What We Review
Introduction
Enzymes are among the most fascinating molecules in biology. They are crucial for processes such as digestion, metabolism, and even DNA replication—essentially any chemical reaction that keeps living organisms functioning. But what exactly makes enzymes so special, and are they truly considered catalysts? In this article, we’ll explore how enzymes work, why they’re classified as catalysts, and how understanding them can help you excel in your AP® Biology studies.
Understanding Enzymes
A. Definition of Enzymes
Enzymes are protein molecules (though some RNA molecules can act as enzymes, called ribozymes) that speed up biochemical reactions in living organisms. They’re vital because they help reactions proceed quickly and efficiently at relatively low temperatures.
B. Basic Structure of Enzymes
- Active Site
- The active site of an enzyme is the region where the substrate—the molecule the enzyme acts upon—binds. This site’s specific shape and chemical environment determine the enzyme’s unique function.
- Enzyme-Substrate Complex
- When a substrate binds to an enzyme’s active site, they form a temporary enzyme-substrate complex. This interaction lowers the energy needed for the reaction to proceed.
C. Types of Enzymes
Enzymes come in many forms, each tailored to a specific function. Examples include:
- Digestive enzymes (e.g., amylase for breaking down carbohydrates)
- Metabolic enzymes that help extract energy from nutrients
- DNA replication and repair enzymes (e.g., DNA polymerase)
Enzymes as Catalysts
A. Definition of Catalysts in Biological Terms
A catalyst is any substance that speeds up a chemical reaction without being consumed or permanently altered in the process. In biological systems, enzymes serve exactly this role.
B. Comparison Between Enzymes and Other Types of Catalysts
Unlike inorganic catalysts (such as metals or chemical compounds in industrial processes), enzymes are highly specific and often work under mild, biological conditions (body temperature, neutral pH, etc.).
C. Explanation of the Catalytic Action of Enzymes
- Mechanism of Action: Lowering Activation Energy
- All chemical reactions require a certain amount of energy to get started, known as activation energy. Enzymes lower this barrier, allowing reactions to occur more readily.
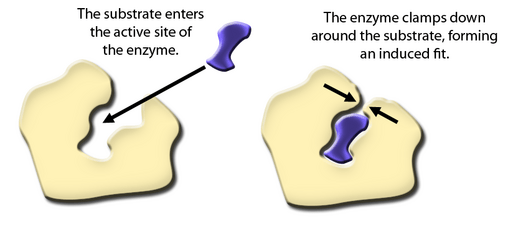
- Specificity of Enzymes (Lock and Key vs. Induced Fit)
- Lock and Key Model: Enzymes have an active site shape that exactly matches a specific substrate.
- Induced Fit Model: The enzyme’s active site may slightly change shape to accommodate the substrate more snugly, enhancing the reaction.
How Do Enzymes Increase the Rate of Reaction?
A. Importance of Activation Energy in Chemical Reactions
Without enzymes, many essential biochemical reactions would be too slow to sustain life. Lowering activation energy is key to making these reactions efficient.
B. How Enzymes Lower Activation Energy
- Transition State Stabilization
- Enzymes stabilize the transition state (the intermediate form between reactants and products), lowering the energy required to reach this state.
- Proximity and Orientation Effects
- By bringing substrates into close proximity and aligning them properly, enzymes increase the likelihood of a successful reaction.
C. Examples of Enzyme-Catalyzed Reactions and Their Rates
- Catalase breaks down harmful hydrogen peroxide into water and oxygen extremely fast—thousands of molecules per second.
- DNA polymerase helps replicate DNA at high speed while maintaining accuracy, vital for cell division.
Factors Affecting Enzyme Activity
A. Temperature
Most enzymes function optimally at around body temperature (37°C). Higher temperatures can denature enzymes, while lower temperatures slow them down.
B. pH Levels
Many enzymes work best at a specific pH range. For example, pepsin (a digestive enzyme in the stomach) requires a strongly acidic environment.
C. Enzyme Concentration
Increasing enzyme concentration typically speeds up the reaction, provided there’s enough substrate available.
D. Substrate Concentration
If the substrate level is too low, not all enzymes can bind a substrate at a given time, limiting the reaction rate.
E. Presence of Inhibitors and Activators
- Inhibitors reduce enzyme activity by blocking or altering the active site.
- Activators can bind to the enzyme to increase its activity.
Real-World Applications of Enzymes
A. Role in Digestion and Metabolism
Enzymes such as amylase, lipase, and proteases break down complex food molecules into simpler compounds that your body can absorb.
B. Industrial Applications
Enzymes are widely used in food production (e.g., cheese-making, brewing), pharmaceuticals (for drug synthesis), and even in detergents to help remove stains.
C. Enzymes in Biotechnology and Environmental Applications
Scientists utilize enzymes in genetic engineering (e.g., restriction enzymes for DNA analysis) and pollution control (enzymes that break down contaminants).
Practice Questions
A. Multiple-Choice
- What is the primary function of an enzyme?
- Slowing down reactions
- Increasing activation energy
- Speeding up reactions by lowering activation energy
- Completely changing itself during the reaction
- Which of the following describes the induced fit model of enzyme activity?
- Perfectly shaped active site with no flexibility
- The active site becomes more flexible to snugly fit the substrate
- Enzymes require high temperatures to function
- Substrates are changed into enzymes
B. Short-Answer
- Explain how enzyme specificity is related to its active site. Provide an example of lock and key versus induced fit.
C. Scenario
- A student conducts an experiment with the enzyme lactase, varying pH levels. Predict how pH may affect the enzyme’s rate of reaction.
Conclusion
Enzymes are indeed catalysts—biological catalysts that lower the activation energy of reactions, thus dramatically increasing their rates. Mastering how enzymes work is an essential step in AP® Biology. You’ll see them come up in questions about metabolism, cell signaling, and genetics. Delving deeper into real-life examples or conducting simple enzyme experiments (like observing how temperature affects catalase in potato) will strengthen your grasp of this topic.
Sharpen Your Skills for AP® Biology
Are you preparing for the AP® Biology test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world math problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
Need help preparing for your AP® Biology exam?
Albert has hundreds of AP® Biology practice questions, free response, and full-length practice tests to try out.








