Introduction
There are many topics that might not appear on the AP® Biology exam, but one thing you can be certain of is that you will be expected to know the main facts about the three groups of important macromolecules: carbohydrates, proteins, and lipids. In this AP® Bio Crash Course Review, we will help you review what you need to know about carbohydrates in particular. We’ll start with some basic definitions about what carbohydrates are, then move on to some more detail about the three classes (monosaccharides, disaccharides, and polysaccharides). We’ll then look at the four main types of polysaccharides – where to find them, how to differentiate them, and why they are important – before finally moving on to some review questions and a quick recap. By the end of this AP® Biology Crash Course Review, you should feel confident in your knowledge about carbohydrates for the AP® Bio exam.
What is a Carbohydrate?
Let’s start with a carbohydrate definition. Carbohydrates, or carbs as they are known colloquially, are energy storing organic compound molecules made up of carbon, hydrogen, and oxygen in a 1:2:1 ratio. Because of this, all carbohydrates follow the empirical formula of { C }_{ n }{ H }_{ 2 }O. All carbohydrates are made up of sugar subunits or monomers. Since they are very large molecules formed by joining up smaller molecules, carbohydrates belong to the family of macromolecules.
From a biological standpoint, the main purpose of carbohydrates is that the body can use them as a form of quick energy. Foods that are high in carbohydrates include pasta, bread, rice, and foods high in sugar like cookies and candy. This is the reason that runners and other athletes are encouraged to carbo-load the night before big matches and events by having pizza and pasta parties. As they load up on lots of carbohydrates the evening before, they will have quick sources of energy ready to use the next day when they need it most.
There are three different classes of carbohydrates, based on the number of sugar subunits they have.
The Three Classes of Carbohydrates
Monosaccharides, as you can guess by the prefix mono-, meaning one, these carbs have a single sugar subunit or monomer. The three main examples of monosaccharides are glucose, fructose, and galactose, some of which you may recognize as the forms of sugar you find on the ingredients lists of many of the foods you eat. Because they are all isomers of each other, the chemical formula for all three of them is { C }_{ 6 }{ H }_{ 12 }{ O }_{ 6 }, although they have different structures.
Disaccharides, as you can guess by the prefix di-, meaning two, have two sugar subunits. The main types of disaccharides to know are maltose, lactose, and sucrose. Again, you’ve likely seen these terms before on the contents lists of foods you eat every day. Sucrose for example, is simple table sugar, while lactose is the sugar found in milk (this is the sugar to which people who are lactose intolerant have negative reactions).
To form a disaccharide, two monosaccharides are joined through a process called dehydration synthesis, or condensation. Just like the condensation you are familiar with in everyday life, this process involves the removal of a water molecule. Because of this, the chemical formula for disaccharides is { C }_{ 12 }{ H }_{ 22 }{ O }_{ 11 } instead of { C }_{ 12 }{ H }_{ 24 }{ O }_{ 12 }, because one { H }_{ 2 }O molecule was lost to condensation when the two monomers were bound. When the two monosaccharides are joined through this process, a covalent bond is formed between them, which is known as glycosidic linkage. The reverse process of dehydration synthesis is called hydrolysis, so when water is added to a disaccharide, it produces two monosaccharides.
The third type of carbohydrates consists of polymers known as polysaccharides. As the prefix poly– might suggest, these are formed when multiple monosaccharides join together (again through the process of dehydration synthesis) to form a polymer with long sugar chains. There are four important types of polysaccharides that you will need to know, which we will go over in the next section.
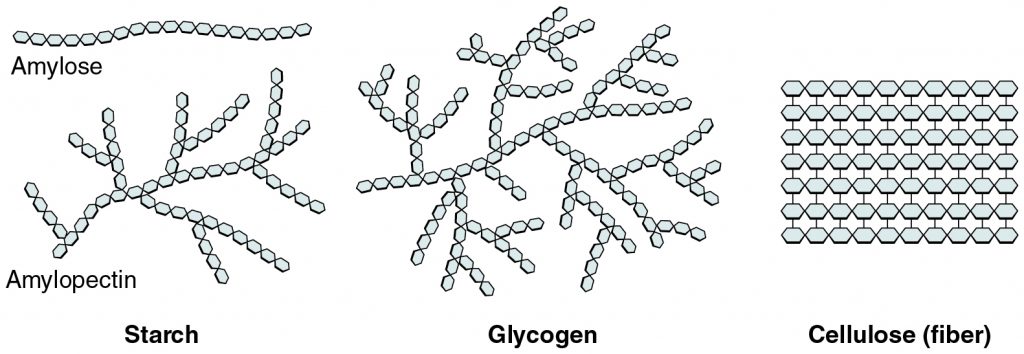
Important Polysaccharides
Polysaccharides serve important functions with regards to cell structure and storage in both plants and animals.
In plants, cellulose is essential for making cell walls and providing structure. Starch is necessary for sugar storage and has two primary forms: amylose and amylopectin. Both starch and cellulose are made up entirely of glucose monomers that are joined together through glycosidic linkages, although the specific types of linkages differ. While starch is formed with alpha-glycosidic bonds, which can be broken down by the enzymes in the digestive tracts of animals, cellulose is formed with beta-glycosidic bonds, which cannot be broken down by animals. This is why we cannot eat certain parts of plants, like wood, because, although they are full of glucose, it is not in a form that we are able to digest.
In animals, glycogen is important for storage and is sometimes known as “animal starch.” It is stored in the liver and skeletal muscles as glucose that can be quickly broken down and used for energy. Some animals also use a polysaccharide called chitin for structure. Chitin makes up the exoskeletons of arthropods (think of beetles and lobsters) and also makes up the cell walls of fungi. Like cellulose, chitin is formed with beta-glycosidic bonds and thus cannot be digested by animals.
Review Questions
Question 1: Which of the following is the chemical reaction that makes polymers by joining monomers?
A) Hydrolysis
B) Ionic bonding
C) Dehydration synthesis
D) Evaporation
E) Protein synthesis
Question 2: Amylase is an enzyme in animals that is used to break down alpha-glycosidic linkages. Which of the following would amylase be able to break down during digestion?
A) Cellulose
B) Glycogen
C) Chitin
D) All of the above
E) None of the above
Question 3: A molecule that has the chemical formula of { C }_{ 6 }{ H }_{ 12 }{ O }_{ 6 } could be which of the following?
A) Sucrose
B) Fructose
C) Lactose
D) Glucose
E) Both A and C
F) Both B and D
Question 4: True or False – the following formula demonstrates the process of dehydration synthesis: Sucrose + Water → Fructose + Glucose
A) True
B) False
Answers
Question 1. The correct choice is option C – dehydration synthesis. This is the formation of a covalent bond between two monomers through the loss of a water molecule. It is also known as condensation.
Question 2. The correct answer is B – glycogen. Animals are not able to break down cellulose or chitin since they are bonded with beta-glycosidic linkages. Don’t let the “all/none of the above” options throw you off!
Question 3. The correct answer is F – both B and D. Fructose and Glucose are both monosaccharides and are isomers, meaning they have the same chemical formula ({ C }_{ 6 }{ H }_{ 12 }{ O }_{ 6 }). Sucrose and Lactose are disaccharides.
Question 4. The correct choice is B – False. The formula shows the opposite of dehydration synthesis, known as hydrolysis. Water is being added to a disaccharide to break it down into to monosaccharides. It would be true if it were the other way around.
AP® Biology Crash Course Recap
We’ve gone over much more than just a simple carbohydrate definition, so review the following AP® Bio Crash Course info to make sure you can really answer the question, what is a carbohydrate?
- Carbohydrates are macromolecules made up of carbon, hydrogen, and oxygen and follow the formula of { C }_{ n }{ H }_{ 2 }
- There are three classes of carbs: monosaccharides, disaccharides, polysaccharides
- Monosaccharides are simple sugars made of one monomer: glucose, fructose, galactose
- Disaccharides are made of two monomers: sucrose, lactose, maltose
- Monosaccharides are joined through glycosidic linkage
- There are four main polysaccharides, shown in the table below:
| Animals | Plants | |
| Storage: | Glycogen | Starch |
| Structure: | Chitin | Cellulose |
- Cellulose and Chitin are bound through beta-glycosidic bonds and cannot be digested by animals
- Starch and Glycogen store glucose for future energy use
Now you know what carbohydrates are and why they are important for all living things. They are only one of the major organic compounds you will need to know for AP® Biology, however. Make sure you also go over lipids, proteins, and nucleic acids as well.
Need help preparing for your AP® Biology exam?
Albert has hundreds of AP® Biology practice questions, free response, and full-length practice tests to try out.
Are you a teacher or administrator interested in boosting AP® Biology student outcomes?
Learn more about our school licenses here.








