What We Review
Introduction
Welcome to your AP® Biology review on common macromolecule monomers! Understanding these basic building blocks is key to grasping how larger biological molecules form and function. From nucleic acid monomers that store genetic information to carbohydrate monomers that provide energy, and from protein monomers involved in countless cellular processes to lipids monomers essential for cell membranes—each of these common macromolecule monomers holds an important place in the world of biology.
Overview of Macromolecule Monomers
Macromolecules are large, complex molecules vital to life. They are typically formed by smaller subunits called monomers. The four major types of biological macromolecules are:
- Carbohydrates
- Proteins
- Lipids
- Nucleic acids
Each type of macromolecule has its own monomer (carbohydrates monomer, protein monomer, lipids monomer, nucleic acid monomer) and plays unique roles in organisms.
Nucleic Acid Monomers
A. Structure of Nucleotide Monomers
Nucleic acids such as DNA and RNA are made of repeating units called nucleotides. Each nucleotide has three components:
- A five-carbon sugar (deoxyribose in DNA, ribose in RNA)
- A phosphate group
- A nitrogenous base (adenine, guanine, cytosine, thymine in DNA; uracil replaces thymine in RNA)
In a simplified diagram, you might imagine the sugar ring in the middle, the phosphate group (P) branching off one side, and the nitrogenous base branching off the other side.
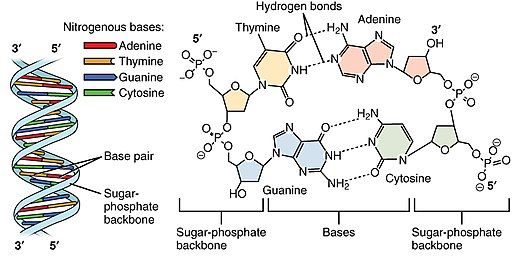
B. DNA vs. RNA
- DNA (deoxyribonucleic acid): Double-stranded, contains the bases A, T, G, and C. The sugar is deoxyribose. It stores genetic information used in development and functioning.
- RNA (ribonucleic acid): Generally single-stranded, contains the bases A, U, G, and C. The sugar is ribose. It helps in protein synthesis (mRNA, tRNA, rRNA) and can have regulatory roles in the cell.
Protein Monomers
A. Structure of Amino Acid Monomers
Proteins consist of amino acid monomers linked together. Each amino acid shares a common structure:
- An amino group (–NH₂)
- A carboxyl group (–COOH)
- A unique side chain (R group) that varies among different amino acids
Some R groups are hydrophobic, some hydrophilic, and some even have ionic charges, all affecting how the protein folds and functions.
B. Primary Structure and Function
The specific sequence of amino acids in a polypeptide chain is referred to as the primary structure. This sequence dictates how the polypeptide will fold into a three-dimensional shape, ultimately determining the protein’s function. For example, enzymes must fold correctly to form an active site that can bind substrates and catalyze reactions efficiently.
Carbohydrate Monomers
A. Structure of Sugar Monomers
Carbohydrates monomer units are monosaccharides (simple sugars) such as glucose, fructose, and galactose. These sugars typically follow a ring structure in aqueous solutions.
B. Complex Carbohydrates
Polysaccharides like starch, glycogen, and cellulose form when multiple sugar monomers link together. Variations in the bonding patterns of monosaccharides lead to diverse functions:
- Starch and glycogen: Energy storage in plants and animals, respectively.
- Cellulose: Structural support in plant cell walls.
Lipid Monomers
A. Structure of Fatty Acids
Lipids consist of fatty acid monomers attached to glycerol backbones. Fatty acids can be:
- Saturated: No double bonds (solid at room temperature).
- Unsaturated: One or more double bonds (liquid at room temperature).
B. Phospholipids and Their Importance
Phospholipids, major components of cell membranes, have a glycerol backbone, two fatty acid “tails” (hydrophobic), and a phosphate-containing “head” (hydrophilic). This dual nature (polar heads, nonpolar tails) allows phospholipids to form bilayers, creating selective barriers around cells.
Types of Bonds Connecting Monomers
A. Overview of Bond Types
Monomers connect through covalent bonds, forming stable long chains (polymers). Key bond types you should know:
- Phosphodiester bonds: Link nucleotides in DNA and RNA.
- Peptide bonds: Connect amino acids into polypeptides.
- Glycosidic linkages: Join monosaccharides in carbohydrates.
- Ester bonds: Join fatty acids to glycerol in lipids.
B. Importance of Bonding in Macromolecule Function
The nature and positioning of these bonds greatly affect macromolecule stability and function. For instance, a slight change in an amino acid’s position (peptide bond arrangement) can alter a protein’s function entirely.
Conclusion
An understanding of common macromolecule monomers—nucleic acid monomers, protein monomers, carbohydrate monomers, and lipid monomers—is central to unraveling major biological processes. Mastering how these molecules bond, fold, and interact will give you a strong foundation for the AP® Biology exam and beyond!
Practice Problems and Examples
- Identify the Monomer:
- A molecule with a five-carbon sugar, a phosphate group, and a nitrogenous base
- A sugar ring with multiple hydroxyl groups (–OH)c) A molecule with an amino group, carboxyl group, and varying R group
- Explain the Role of Bond Types:– How would you differentiate between a phosphodiester bond and a peptide bond? Provide examples of where each is found in a cell.
- Compare and Contrast:– How does the structure of saturated vs. unsaturated fatty acids affect the physical properties of lipids?
Illustrative Diagram Tips (imagining or drawing at home):
- For a nucleotide: Draw a pentagon (sugar) connected to a circle (phosphate) and a rectangle/hexagon (base).
- For an amino acid: Draw a central carbon with four different substituents—H, NH₂, COOH, and an R group.
- For simple sugars: Show a ring structure with carbons labeled and hydroxyl groups attached.
By working through these questions and revisiting the basic structures, you’ll reinforce your understanding of how monomers come together to form the essential macromolecules of life. Best of luck with your studies—stay curious and keep exploring!
Sharpen Your Skills for AP® Biology
Are you preparing for the AP® Biology test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world math problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
Need help preparing for your AP® Biology exam?
Albert has hundreds of AP® Biology practice questions, free response, and full-length practice tests to try out.