What Is Dehydration?
When you hear the word dehydration, you undoubtedly think back to the last time you felt thirsty, dizzy, and generally off after a major loss of water (and insufficient water intake). Though we typically view the word dehydration in a negative light, the concept of dehydration synthesis is extremely valuable at the molecular level.
In biology and chemistry, a dehydration synthesis reaction (or a condensation reaction) is one that synthesizes—or joins—two molecules together, resulting in a loss of water.It may not sound like much, but the underlying importance of dehydration synthesis is that it is central to the production of larger biological molecules to be used in other biological processes.
We’ll talk a little bit later about dehydration synthesis as a process for the formation of these biological macromolecules (in the “Formation of Polymers/Biological Macromolecules” section of this AP® Biology Crash Course Review). For now, just know that dehydration reactions and their products are key to the performance of many biological functions. Without dehydration synthesis, life as we know it could not exist.
Now is a good time to mention hydrolysis, which is the opposite of dehydration synthesis. Rather than joining molecules together and releasing water as a product, hydrolysis uses water to split apart large molecules into their respective components. If you want to throw in some dry science humor, you might even say that hydrolysis is the “equal but opposite reaction” to dehydration synthesis.
To learn more about how hydrolysis works and how it functions in biological processes, look at our Hydrolysis AP® Biology Crash Course, if you haven’t already.
Before we get any further into this AP® Biology Crash Course Review and look at how dehydration synthesis works, let’s take a moment to review exactly why water is so important to biological life (and why it shows up in so many chemical reactions).
The Importance of Water

Think back again to how you felt the last time you really needed a drink of water—that thirst is your body’s way of telling you it needs H2O for its chemical processes… but why? As you may have previously learned, water is arguably the most versatile, useful chemical in all of biology.
If you’ve read our Hydrolysis AP® Biology Crash Course, you know that water is a polar molecule. Because of the charges at work, the two hydrogen atoms and one oxygen atom in a water molecule are arranged in a familiar mouse-like fashion, sporting what looks like a head (the oxygen atom) and ears (the hydrogen atoms). Due to this arrangement, the overall molecule has a distinct negatively-charged end and a distinct positively-charged end. These ends are referred to as poles, just as our planet has the North and South poles on opposite ends of the planet.
Thanks in part to its polar structure, water boasts a set of special properties that make it biologically valuable. One such property is the presence of weak hydrogen bonds between water neighboring molecules, which play into many of water’s special characteristics, including its high heat capacity.
In the case of dehydration synthesis, water is simply released as a product and is not used as an input. Nevertheless, the water that is produced via a dehydration reaction is important in that it can be used for other reactions in the body. No other chemical is as critical to the success of living things.
Key Chemistry Terms
To understand dehydration synthesis at a molecular level, there are a few key chemistry terms with which you’ll want to familiarize yourself:
• Reactant: a chemical that serves as an input to a reaction.In the reaction a + b = c, a and b would be considered the reactants. In a dehydration synthesis reaction, the original molecules being joined are considered the reactants.
• Product: the output of a chemical reaction.In the reaction a + b = c, c would be considered the product (note: this example is simplified; a reaction may have multiple products).In a dehydration synthesis reaction, the products include the final conjoined molecule and the released H2
• Hydroxyl group: a bonded oxygen and hydrogen group that is attached to a given molecule. These appear as “OH” groups in molecular diagrams.
The Chemistry of Dehydration Synthesis
As mentioned briefly before, two primary things occur in every dehydration synthesis:
1. Two reactants combine to form a new product.
2. Water is lost as a result of the reaction.
The process sounds fairly straightforward, and it really is quite chemically simple at its core. Take a look at the following diagram of the dehydration synthesis of sucrose from its constituent molecules, glucose, and fructose.
Formation of Polymers/Biological Macromolecules
We all see plastics at work nearly every day of our lives. They take thousands of different shapes to serve a wide variety of purposes, from those colorful sturdy building blocks to stretchy, seemingly-unbreakable trash bags. But have you ever wondered how plastic products manage to be so versatile and strong?
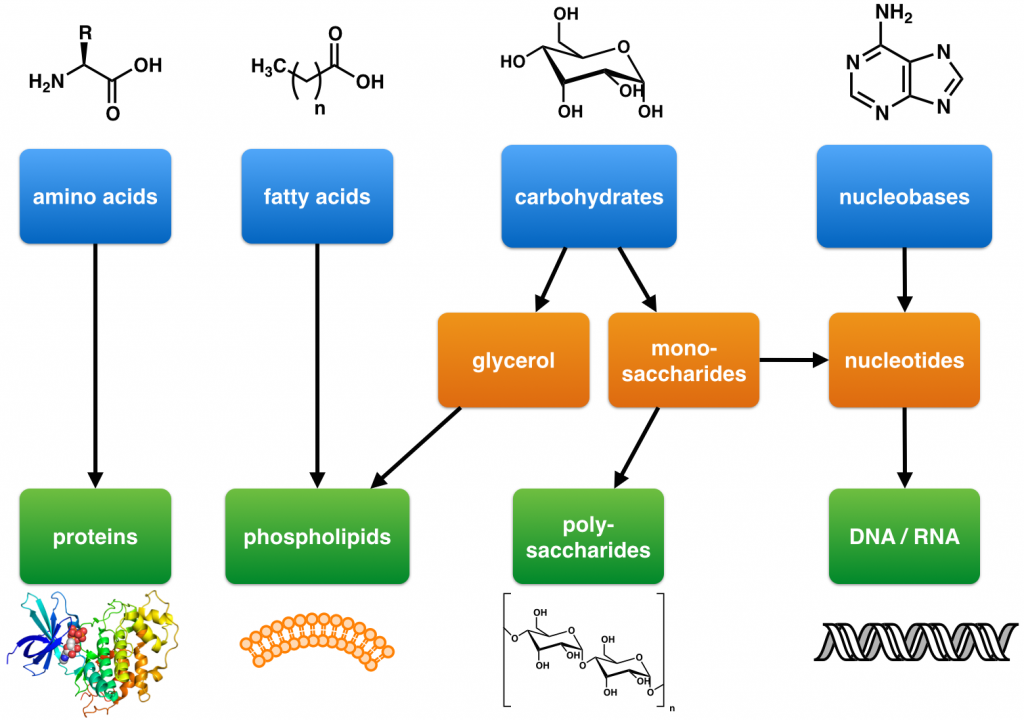
The answer lies in the formation of polymers. As you might have guessed from the poly prefix, polymers comprise multiple molecules joined into a repeating chain or branching network. Polymers can be either natural (like proteins) or synthetic (like plastics) and exhibit a wide range of characteristics that make them useful in both biological and non-biological tasks.
In biology, some of the most common polymers—or biological macromolecules—are not plastics, but various carbohydrates, lipids (fatty acids), proteins, and nucleic acids (DNA and RNA).
In our previous example, starring fructose, glucose, and sucrose, sucrose is considered a polymer (called a disaccharide due to its two simple sugar components). By contrast, the initial fructose and glucose molecules are monomers (hence the name monosaccharides). Disaccharide sugars, like sucrose, are carbohydrates and, as they are used in biological processes, are also considered biological macromolecules.
If you’d like to delve a little deeper into the formation and characteristics of polymers, head over to our Polymers AP® Biology Crash Course Review when you’re done here.
AP® Biology Crash Course Review & Practice
Let’s review some highlights of what we’ve learned so far:
• Dehydration synthesis is a reaction that combines molecules and results in a loss of water.
• Water(H2O) is perhaps the most important chemical in all of biology and boasts unique properties that make it versatile and valuable.
• In a dehydration reaction, either a hydroxyl group from one molecule combines with a hydrogen atom from the other molecule, or two hydrogen atoms from one molecule combine with an oxygen atom on the other molecule. In either case, water is released, and the two molecules are joined together.
• Dehydration synthesis is a key step in the formation of polymers, large molecule chains formed by linking together multiple monomer units (via dehydration, of course).
• In biology, dehydration synthesis forms large polymers commonly referred to as biological macromolecules. These include molecules like proteins, lipids (fatty acids), nucleic acids (DNA and RNA), and carbohydrates.
• The opposite process of dehydration synthesis is hydrolysis, in which larger molecules are split into their component parts via a reaction with water.
Think you’re ready to tackle dehydration synthesis on the AP® Bio Test? Try out this practice question, first:
Q: What is the overall biological importance of dehydration synthesis reactions?
A: In addition to joining molecules and forming new products like alcohols and ethers, dehydration synthesis is a process that helps serve as a chemical basis for the building of larger macromolecules.
Need help preparing for your AP® Biology exam?
Albert has hundreds of AP® Biology practice questions, free response, and full-length practice tests to try out.
Are you a teacher or administrator interested in boosting AP® Biology student outcomes?
Learn more about our school licenses here.








