What We Review
Introduction
Enzymes are proteins that catalyze biochemical reactions, speeding up critical processes in cells. In AP® Biology, understanding how enzymes function—and especially how they can become denatured—is essential for grasping how various biological systems work. This article explains how enzymes become denatured, what causes denaturation, and why it matters for students preparing for the AP® Biology exam.
What Is a Denatured Enzyme?
Enzymes, like most proteins, have a complex three-dimensional structure that is crucial for their function. When an enzyme denatures, its three-dimensional structure unravels, causing it to lose its functional shape. Without this proper shape, the enzyme’s active site—which binds to substrate molecules—no longer fits the substrate correctly, leading to a loss of catalytic activity.
What Happens to an Enzyme When It Is Denatured?
- Disruption of Structure:
- During denaturation, the breakdown of hydrogen bonds, ionic bonds, and disulfide bridges disrupts the enzyme’s folded shape.
- Loss of Activity:
- Because the enzyme can no longer bind to its substrate effectively, its catalytic activity declines significantly or disappears altogether.
Environmental Factors Leading to Denaturation
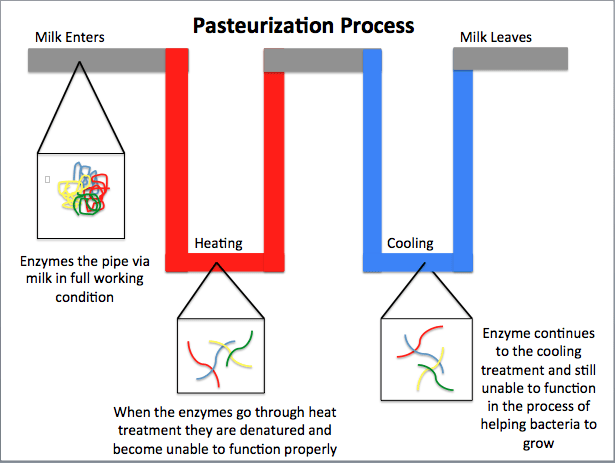
- Temperature: Extremely high temperatures can break the delicate bonds holding the enzyme’s shape, leading to denaturation.
- pH: Each enzyme functions optimally at a particular pH. Deviations from this pH range can alter the charge interactions within the protein, disrupting its structure.
Factors Affecting Enzyme Denaturation
Enzymes generally function within narrow temperature and pH ranges. Even slight shifts can impact activity.
- Temperature:
- Optimal Range: Most enzymes in humans work best at around 37°C (98.6°F).
- Above the Optimal Range: High temperatures increase molecular motion, potentially tearing apart the enzyme’s structure.
- Below the Optimal Range: Although low temperatures slow down reaction rates, they usually do not permanently denature an enzyme; however, extremely low or high temperature fluctuations can sometimes cause irreversible damage.
- pH and Hydrogen Ion Concentration:
- The pH Equation: pH = –log [H+].
- Hydrogen Ion Impact: Changes in [H+] concentration can alter the chemical bonds within the enzyme, especially in the active site. Too many or too few hydrogen ions can disrupt ionic and hydrogen bonds, leading to denaturation.
Reversibility of Enzyme Denaturation
In some cases, mild denaturation can be reversed if conditions return to normal—often called renaturation. This typically happens if the changes in temperature or pH are not too severe or prolonged, allowing the enzyme to refold into its functional shape. However, many instances of denaturation are permanent, especially when the polypeptide chain becomes irreversibly tangled or fragmented.
Practical Applications and Examples
- Biological Systems:
- In our digestive tract, enzymes like pepsin and trypsin have specific pH ranges. If abnormal conditions denature these enzymes, they can impair digestion.
- Industrial and Lab Settings:
- Brewers and manufacturers often monitor temperature and pH to maintain optimal enzyme activity for processes like fermentation. Even slight deviations can ruin a batch of product.
Practice Problems
- Describe one way high temperature causes enzyme denaturation.
- How does pH alteration impact an enzyme’s active site structure?
- If an enzyme is denatured at a temperature slightly above its optimal range, under what conditions might it regain activity?
- Calculate the pH of a solution if the [H+] is 1 × 10⁻⁵ M.
- Provide an example of how a denatured enzyme might affect a specific metabolic pathway in the human body.
Conclusion
Enzyme function is tightly linked to its structure. When denaturation occurs, it often loses or reduces its ability to catalyze reactions. This concept is central in AP® Biology because it illustrates the delicate balance that organisms must maintain to ensure proper enzyme activity. By thoroughly understanding what happens when an enzyme is denatured, students can master a key principle underlying many biological processes.
By exploring these topics and practicing with relevant questions, AP® Biology students can enhance their understanding of enzymes and the critical concept of denaturation, setting a solid foundation for success on the exam.
Sharpen Your Skills for AP® Biology
Are you preparing for the AP® Biology test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world math problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
Need help preparing for your AP® Biology exam?
Albert has hundreds of AP® Biology practice questions, free response, and full-length practice tests to try out.