Density is a fundamental concept in physics that explains how much mass an object contains relative to its volume. For AP® Physics 1 students, mastering the density formula is crucial, as it forms the basis for understanding various physical interactions, particularly in fluids and materials science. This guide aims to simplify the density concept, making it accessible and engaging for high school students.
What We Review
What is Density?
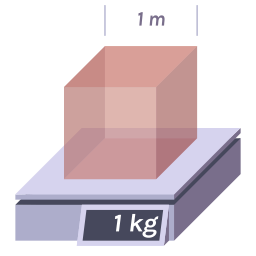
Density is a measure of how compact the mass in a substance or object is, given by the formula \rho = \frac{m}{V} , where \rho is the density, m is the mass, and V is the volume. Mass refers to the amount of matter in an object, measured in kilograms (kg) or grams (g). Volume is the space taken up by an object, with units like cubic meters (m³) or liters (L).
Example: Compare a dense iron cube to a lighter wooden block. Even if both are the same size, iron will have a higher density because it holds more mass in the same volume.
Understanding the Density Formula
The density formula \rho = \frac{m}{V} is fundamental for calculating an object’s density.
- Mass (m): Typically measured in kilograms (kg) or grams (g). This represents how much matter is in an object.
- Volume (V): Often measured in cubic meters (m³) or liters (L), denoting the space the object occupies.
Mastering this relation is important for understanding how materials and fluids behave in various situations.
Example: Calculate the density of a solid object.
- Given: Mass = 30 g, Volume = 10 cm³
- Steps:
- Use the formula: \rho = \frac{m}{V}
- Substitute values: \rho = \frac{30\text{ g}}{10\text{ cm}^3}
- Calculate: \rho = 3 \, \text{g/cm}^3
Density of Common Substances
Materials have different densities, affecting their buoyancy and stability. For instance, water’s density is approximately 1 g/cm³, making it a standard for comparison.
- Metals: Typically have higher densities, explaining why most sink in water.
- Gases: Usually less dense than liquids and solids, hence they spread out and fill spaces.
Example: A solid metal will sink in water if its density is greater than that of water. However, a plastic ball will float because its density is lower.
Characteristics of Fluids
Fluids are substances that flow, including liquids and gases. Unlike solids, fluids do not have a fixed shape.
- Liquids can fill containers to a level and have a defined volume.
- Gases expand to fill the entirety of their containers.
Ideal Fluids: They are assumed to be incompressible and lack viscosity, making calculations simpler in theoretical scenarios. Understanding fluid density aids in predicting how substances behave, like why cream rises on milk.
Calculating Fluid Density
To find the density of fluids, the same formula applies: \rho = \frac{m}{V} .
Example: Calculate the density of a fluid.
- Given: Mass = 50 g, Volume = 40 mL
- Steps:
- Use the formula: \rho = \frac{m}{V}
- Substitute values: \rho = \frac{50\text{ g}}{40\text{ mL}}
- Calculate: \rho = 1.25 \, \text{g/mL}
Conclusion: Density in AP® Physics 1
Density is a fundamental concept in AP® Physics 1 with wide-ranging applications in engineering, meteorology, and oceanography. It determines material properties, fluid behavior, and atmospheric changes, making it essential for analyzing real-world physical systems.
Key Takeaways:
- Density Formula: rho = \frac{m}{V}– Understanding how mass and volume interact is crucial.
- Real-World Applications: From structural integrity in buildings to weather predictions and ocean currents, density influences various fields.
- Practice for Mastery: Solving density problems and exploring its role in different contexts will reinforce your understanding.
To excel in AP® Physics 1, apply density concepts to practical examples, analyze experimental data, and use problem-solving strategies. The more you engage with these ideas, the stronger your physics foundation will become!
| Term | Definition |
| Density (\rho) | Mass per unit volume of a substance. |
| Mass (m) | The quantity of matter in an object. |
| Volume (V) | The space occupied by a substance. |
| Ideal Fluid | A fluid that is incompressible and has no viscosity. |
| Fluid Density | The density characteristic of liquids and gases. |
Sharpen Your Skills for AP® Physics 1
Are you preparing for the AP® Physics 1 test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world physics problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
Need help preparing for your AP® Physics 1 exam?
Albert has hundreds of AP® Physics 1 practice questions, free response, and full-length practice tests to try out.