Introduction
Diffusion and osmosis are two of those mechanisms that you will see pop up over and over again in your biology lessons as they are crucial to so many aspects of life. Throughout this quick crash course, we’ll give you the run-down of all the essential points you’ll need to know not just to prepare for the AP® Biology exam, but also to better understand the ‘big picture’ of how biology works. We’ll start with the basics of diffusion, then move on to osmosis and the different types of osmotic solutions, and end with how those influence plant and animal cells. Finally, we’ll wrap up with some review questions and a quick recap of what we’ve covered. So, let’s get to it!
What is Diffusion?
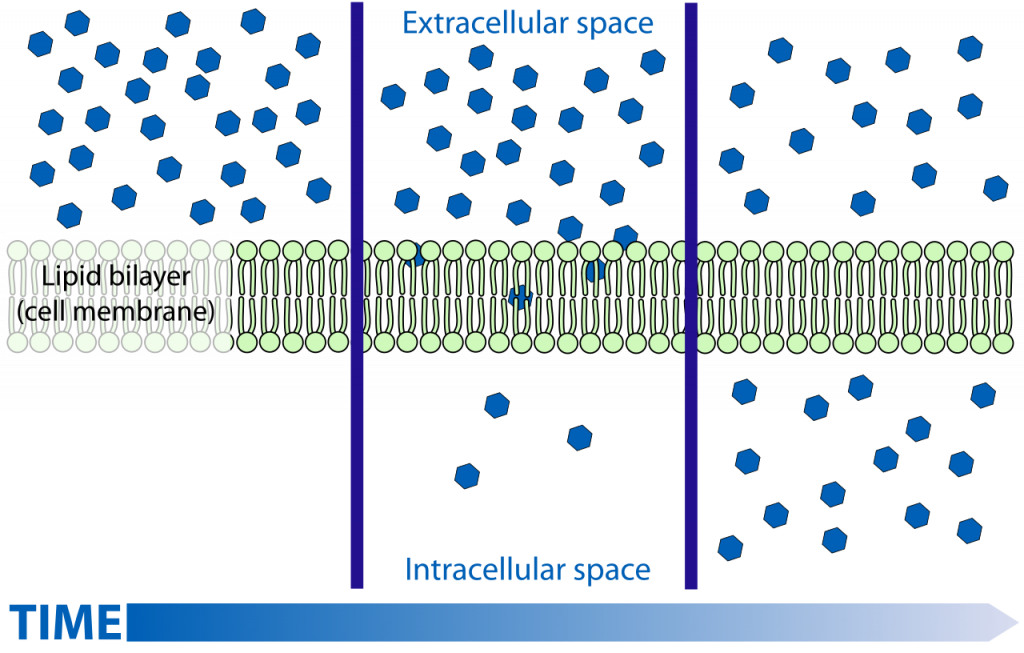
The concept of diffusion is relatively simple and one you see in often in your everyday life. From when you wake up in the morning and add sugar or milk to your coffee, to when you open your window in the afternoon to get that strange smell out of your room, diffusion is a constantly occurring part of your life. In science-y terms, diffusion is the passive movement of molecules from an area where they are highly concentrated to areas where they are less concentrated.
Let’s break that down by looking at the example of adding milk to your coffee. Inside the milk carton, there is equilibrium (at least in the bit where there is milk, let’s not worry about the air that might be in the carton as well). The distribution of the milk and all the molecules it’s made up of is equal and evenly spaced– there is not one side or one corner of the box that has a greater concentration of milk than the other. When you pour some of that milk into your coffee, however, all of a sudden you have these drops of milk that are surrounded by all this non-milky space (i.e., the coffee). There is no longer equilibrium: those drops are areas of high milk concentration surrounded by areas of low milk concentration. As you already know, they don’t just stay as this weird ball of milk in the middle of your coffee, but they spread out until the amount of milk is even throughout your cup, and there is once again equilibrium.
It’s as easy as that. Molecules will move down a concentration gradient from high concentrations to low concentrations until equilibrium is reached. As it says in the definition above, this is a passive transport mechanism, which means that it does not require any energy. When the opposite case is true – molecules moving from low concentrations to high concentrations – energy is required and this is called active transport. There are also some situations, however, when diffusion does need a little bit of extra help, called facilitated diffusion, but before we can get into that, we need to understand a few more concepts:
Membranes and Permeability
Unfortunately, life isn’t always as simple as pouring milk into your coffee. Sometimes there are barriers, and in biology, barriers tend to be membranes. Now generally, the purpose of having a barrier is either to keep stuff in, or to keep stuff out, but, it’s very rare that you’ll want to keep everything in or out. When you have a membrane that allows the passage of some things but not others, that membrane is said to have selective permeability or to be semipermeable.
This is true of all cell membranes, which tend to allow the passage of lipids, gasses, but do not allow proteins or ions or large polar molecules. Perhaps the most well known semipermeable barrier (and an excellent example, should you need to give one on the AP® Biology exam) is the blood-brain barrier (BBB). This is one of the most selective barriers within the human body as it separates the extracellular fluid in your central nervous system from the blood (and all the pathogens in it) circulating in your body. It’s the reason you don’t die as soon as your paper cut gets infected or your tonsils are swollen. Unfortunately, it’s also the reason it’s so hard to treat neurodegenerative diseases – the barrier won’t let through most forms of drugs.
So, how does all this selective permeability affect diffusion? Well, let’s look at cell membranes as an example. As you hopefully already know, cell membranes are made out of a lipid bilayer where the outsides of the membrane are made up of hydrophilic (“water-loving”) heads, and the inside of the membrane is made up of hydrophobic (“water-fearing”) tails. (Note: If this is completely new information to you, make sure you go back and review cell structure – it’s a massive part of AP® bio!). Molecules of water and oxygen are able to flow freely through this membrane on their own through diffusion.
Because of this lipid bilayer, though, other molecules like glucose and sodium ions – both things cells need to function – are unable to pass through on their own. Luckily, cell membranes are also made up of ion channels and carrier proteins that act like gateways along the membrane. The molecules are then able to move in and out of the cell (from higher concentrations to lower) through the process of facilitated diffusion. While some carrier proteins are used in active transport, the examples mentioned here are passive mechanisms that require no energy – just like regular diffusion.
What is Osmosis?
So far, we’ve come to understand the process of diffusion and how it plays into allowing molecules in and out cells in a general sense. Now let’s get a bit more detailed and look at the process of osmosis. Osmosis (for the purposes of the AP® Biology exam) refers specifically to the diffusion of water molecules across membranes. This too is a passive mechanism that requires no energy. For this crash course, it is most relevant to cell membranes.
As per the rules of diffusion, water will always move from higher to lower concentrations. But, for osmosis, sometimes a better way to think of it is that water will always move towards higher concentrations of solutes. One way to remember this is to imagine that water molecules really love salty things. Whenever they find a higher concentration of salt (or whatever other solute) on the opposite side of the membrane they are behind, they will rush through it to get as much salt as they can.
There are different types of solutions describing these situations.
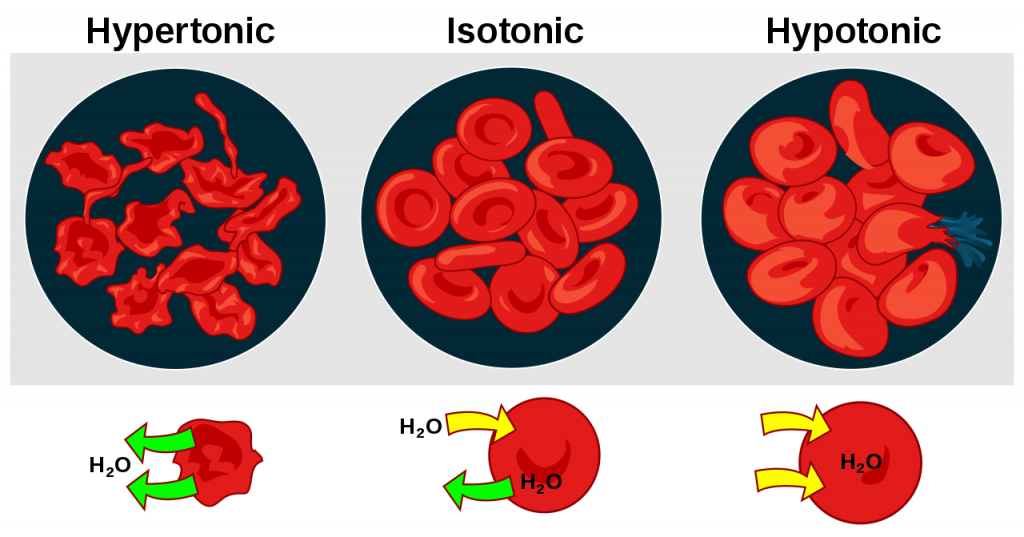
When the concentration of a solute is equal on both sides of a membrane, the two solutions (inside and outside the membrane) are said to be isotonic. This does not mean that there is no movement through the membrane, however, just that the movement is such that the concentrations stay the same. This is called dynamic equilibrium.
When the concentration of a solute is less on one side of a membrane than the other, that solution is said to be hypotonic. The solution on the other side of the membrane (the one with the higher concentration of solute), is said to be hypertonic. Water always moves from hypotonic solutions to hypertonic ones.
Osmosis and Cells
Just like Goldilocks in the story with the three bears, cells are the happiest when the amount of water they have is just right – in other words when their solutions are isotonic.
When a cell is in a hypotonic environment, meaning the environment has a lower concentration of solutes and a higher concentration of water compared to the cell, water will enter into the cell causing it to grow. You can easily observe this for yourself. A plant with lots of hypotonic cells will wilt and shrivel, but as soon as you water it, the cells will become stiff and rigid as they fill with water, allowing the plant to stand tall once again.
In animal cells, organelles known as contractile vacuoles will pump water out of the cell if there is too much coming in. Plants have strong cell walls that will keep them from bursting, but animal cells do not. Because of this, animal cells burst much more easily than plant cells and can be destroyed through a large influx of water. When a cell is destroyed because of the rupture of its cell wall or cell membrane, it is called lysis. The lysis of red blood cells specifically is known as hemolysis.
When a cell is in a hypertonic environment with more solute molecules outside of the cell than in it, water is sucked out of the cell, causing it to shrink or undergo crenation. This is what caused the plant in our previous example to wilt in the first place. If the plant had not been watered, its cytoplasm would have continued to shrink to the point where the cell membrane pulled away from the cell wall, causing it to die. This is called plasmolysis. In times of war, people would often salt the fields of their enemies, ensuring that the environment would be too hypertonic for any crops to be able to grow.
While hypertonicity can be deadly for animals as well – it’s the reason you’re not meant to drink seawater, as it will cause you to dehydrate faster than not drinking anything at all – many animals have developed adaptations to counter its effects. The fish in the ocean, for example, are always in hypertonic solutions, but, seeing as they are always in water, they are able to immediately replace all of the osmotic water loss by continually drinking large amounts and excreting the salt. This is a form of osmoregulation.
Review Questions:
Question 1. Which of the following is true?
- Facilitated diffusion requires more energy than regular diffusion.
- Osmosis requires more energy than facilitated diffusion
- Regular diffusion requires more energy than facilitated diffusion.
- Facilitated diffusion and regular diffusion require the same amount of energy
- Both A and B
Question 2. Explain why saltwater fish need a form of osmoregulation to survive.
Question 3. If someone is admitted to a hospital for severe dehydration, what type of solution should the doctor administer through the IV drip to make him or her feel better?
- One that is hypotonic
- One that is hypertonic
- One that is ultratonic
- One that is isotonic
Answers:
Question 1. The correct choice is option D. Neither facilitated diffusion nor regular diffusion requires more energy than the other, as they are both passive mechanisms. The processes require no energy.
Question 2. The reason saltwater fish need osmoregulation to survive is because their cells are constantly in hypertonic environments. The seawater around them always has higher concentrations of solutes than their cells do. Without osmoregulation, the cells of the fish would dehydrate, as all of the water would be moving out of them.
Question 3. The correct solution is option A, one that is hypotonic. Since the person is very dehydrated, the doctor wants more water to enter the patient’s cells. To do this, the cells need to be in an environment that has a lower concentration of solutes than the cells. Water moves from hypotonic solutions to hypertonic ones.
Crash Course Quick Recap for Diffusion and Osmosis
- Diffusion is the passive movement of molecules down the concentration gradient from areas of high concentration to low concentration
- Osmosis is the diffusion of water molecules across a membrane
- Cell membranes are semipermeable, only allowing select molecules to pass through
- Some molecules pass through membranes with help from ion channels or carrier proteins in a process called facilitated diffusion
- There are three types of osmotic solutions: isotonic, hypotonic, and hypertonic
Need help preparing for your AP® Biology exam?
Albert has hundreds of AP® Biology practice questions, free response, and full-length practice tests to try out.
Are you a teacher or administrator interested in boosting AP® Biology student outcomes?
Learn more about our school licenses here.








