What We Review
Introduction
Enzymes are at the heart of nearly every biological process, from digesting food to synthesizing essential molecules. Their importance in driving life’s chemical reactions cannot be overstated. In this post, we’ll explore how enzymes function, focusing on enzyme structure and properties—key concepts you need to master for the AP® Biology exam.
What are Enzymes?
Enzymes are specialized proteins that act as biological catalysts. They speed up reactions that would otherwise occur too slowly to sustain life. By lowering the activation energy required for a chemical reaction, enzymes allow organisms to efficiently regulate metabolic pathways. Think of enzymes as the organizers of a busy factory, making sure all reactions proceed quickly and smoothly.
Properties of Enzymes
A. Specificity
Enzymes exhibit remarkable specificity, binding only the substrates that fit both the shape and charge of their active site. For instance, the enzyme lactase specifically targets lactose (the sugar in milk) due to their complementary shapes, much like a lock and key.
B. Efficiency
Enzymes drastically increase the rate of chemical reactions—often by factors of millions. When you compare reaction rates with and without an enzyme, the difference is astounding. This efficiency is essential for cells as it ensures that vital processes happen rapidly.
C. Sensitivity to Environmental Conditions
Environmental factors—particularly temperature and pH—have profound effects on enzyme activity. Each enzyme works best within an optimal range. Outside this range, the enzyme’s structure can unravel (a process called denaturation), preventing it from binding to substrates effectively.
Enzyme Structure
A. General Structure of Enzymes
Like other proteins, enzymes have several levels of structure:
- Primary Structure: The sequence of amino acids.
- Secondary Structure: Folding into α-helices and β-pleated sheets, stabilized by hydrogen bonds.
- Tertiary Structure: The overall 3D shape, crucial for forming the active site.
- Quaternary Structure: The arrangement of multiple polypeptide subunits, if present.
Visualizing these layers can help you understand how even small changes—like a single amino acid substitution—may alter enzyme function.
B. The Active Site
The active site is the pocket or groove on the enzyme where the substrate binds. Its specific arrangement of amino acids fosters the precise interactions needed to catalyze the reaction. Picture this as the perfect workspace designed to support a chemical change.
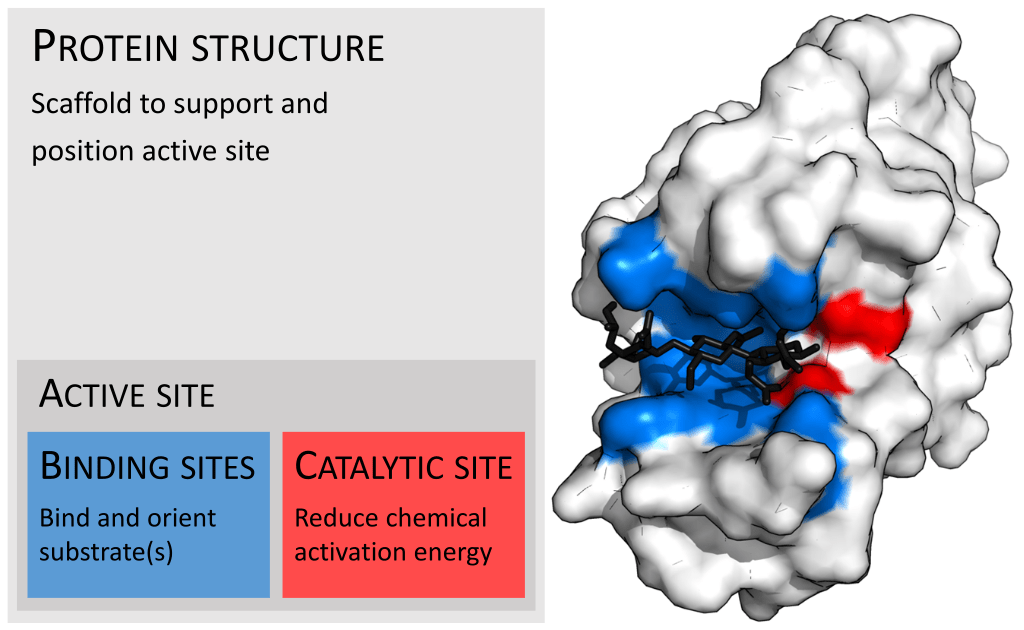
Enzyme-Substrate Interaction
A. Compatibility of Shape and Charge
Effective interaction depends on structural and electrostatic compatibility between the enzyme and substrate. The lock-and-key model suggests that the substrate must fit the enzyme perfectly, whereas the induced fit model explains that enzymes can slightly change shape to accommodate the substrate.
B. Factors Affecting Binding
Hydrogen bonds, ionic interactions, and other non-covalent forces keep substrates firmly in place once they enter the active site. Small changes in environment (temperature, pH, concentration of cofactors) can strengthen or weaken these bonds, influencing the binding efficiency.
Practice Problems
- Define enzyme specificity and provide one example that illustrates this concept.
- How does temperature generally affect enzyme activity, and what happens when an enzyme is exposed to temperatures far above its optimal range?
- Compare and contrast the lock-and-key model and the induced fit model of enzyme action.
- Explain how changing the primary structure of an enzyme might alter its overall function.
Answers and Explanations
- Specificity refers to the unique fit between an enzyme and its substrate due to shape and charge. An example is lactase and lactose, fitting together like a key in a lock.
- Each enzyme has an optimal temperature for activity. Extremely high temperatures can denature the protein, disrupting its 3D structure and stopping its function.
- The lock-and-key model suggests a perfect fit from the start, while the induced fit model proposes that the enzyme adjusts its shape slightly to accommodate the substrate.
- Changing even one amino acid in the primary structure can alter folding in higher structures, potentially destroying the active site and rendering the enzyme nonfunctional.
Conclusion
Understanding enzyme structure is crucial for grasping how these catalysts work. From the shape of the active site to the delicate balance of chemical bonds, every aspect of an enzyme is intricately tuned to perform specific tasks. As you study for the AP® Biology exam, remember that enzymes illustrate one of life’s most essential design principles: form supports function.
By revisiting your practice problems, you’ll build a robust understanding of enzymes—one of the most fascinating topics in AP® Biology. Good luck with your studies and exam prep!
Sharpen Your Skills for AP® Biology
Are you preparing for the AP® Biology test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world math problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
Need help preparing for your AP® Biology exam?
Albert has hundreds of AP® Biology practice questions, free response, and full-length practice tests to try out.