In this AP® Biology Crash Course, we will review what you need to know about enzymes for the AP® Biology exam. We will cover what enzymes are, how enzymes work, some factors that affect how they work, and finally an example of an AP® Bio question about enzymes.
What are Enzymes?
Enzymes are proteins that catalyze chemical reactions. Molecules at the beginning of the chemical reactionary process are called substrates, and these are converted into products. Enzyme kinetics, or Michaelis-Menten kinetics, investigate how enzymes bind substrates and turn them into products. The amount of substrate needed to reach a given rate of reaction is the Michaelis-Menten constant. Almost all metabolic processes require enzymes to occur at the proper rate.
Some chemical reactions take a lot of energy to start. The amount of energy needed to kick off a chemical reaction is called its activation energy. Enzymes help the chemical reaction reach the activation energy by lowering the amount of energy needed to overcome it.
French chemist Anselme Payen discovered the first recognized enzyme, diastase, in 1833. Louis Pasteur also noticed when studying a mixture of sugar, alcohol, and yeast, something was happening to ignite the fermentation process. The word “enzyme” was first used by a German physiologist in 1877 named Wilhelm Kuhne.
Enzyme Structure
As you may have learned in your AP® Biology course, an enzyme’s primary structure is nothing more than a long sequence of amino acids that bond with one another. Short-range interactions (secondary) between amino acids can be alpha-helix or beta sheet. Alphas look like spirals, and betas look like flat, wavy sheets.
The long-range interactions (tertiary) are when amino acids interact with other amino acids a long way down the strand, and as they fold over, they form a globular structure. The quaternary structure is when one globular strand interacts with other tertiary pieces. When bonds are formed at this level, they are often hydrogen bonds, but sometimes it is two hydrophobic pieces interacting, or even ionic bonds. Alternatively, when an enzyme is unfolded, it’s referred to as being denatured.
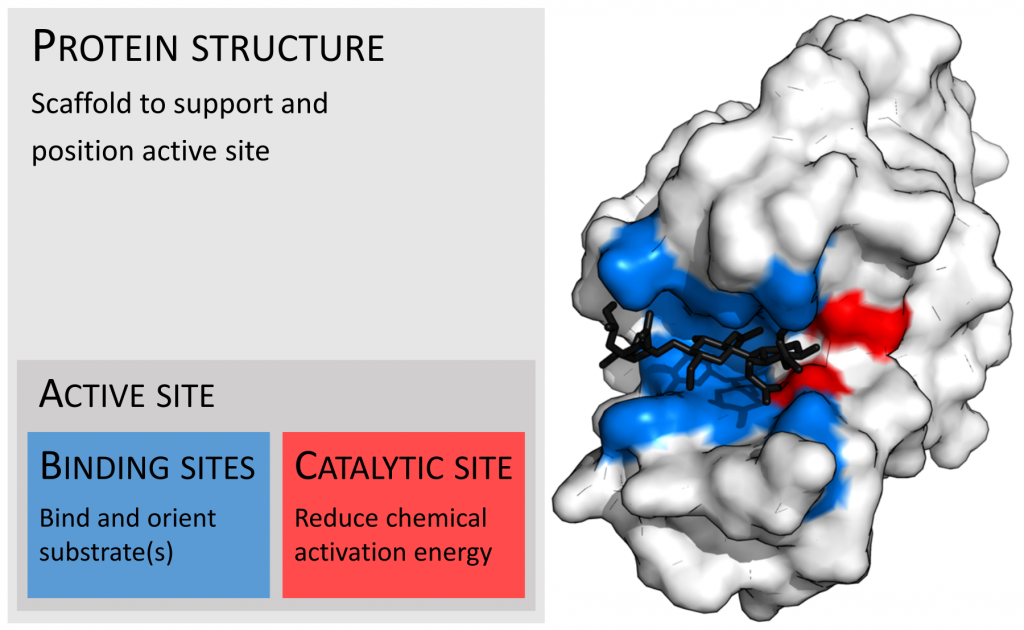
Enzymes are quite large relative to their substrates, yet only a small portion of the structure is involved in the reaction; that part is referred to as the catalytic site. This site is located next to a binding site where residues orient the substrates. These two sites together are referred to as the active site.
Enzyme Activation
In order for an enzyme to work, it must be activated by the binding of another molecule. Activators can either be cofactors or coenzymes; cofactors are small, inorganic chemicals, and coenzymes are organic compounds. Both of these activators bind to the active site but are not considered substrates. When they bind to the active site, there is often a conformation change. A conformation change is a change in the enzyme’s configuration or shape. The change in shape alters the active site and allows the substrate to bind.
How Do Enzymes Work?
Enzymes are extremely selective about which substrates they are able to bind to. Related to the specificity of enzyme and substrate bonding, Emil Fischer proposed the lock and key model where the two would have complementary geometric forms. Daniel Koshland suggested that these complementary geometric pieces can actually shift and can even be reshaped by their interactions with substrates. This new discovery led to the induced fit model.
The induced fit model refers to the ability for the substrate and enzyme to modify their shape in order to fit together. After the enzyme and substrate have bound to each other, the enzyme will work to lower the activation energy of the chemical reaction.
In order to understand how enzymes work, we should review activation energy and Gibbs free energy. Using the Gibbs free energy models, we can see that the energy of the reactants is lower than the activation energy. The activation energy (delta G) is the amount of energy that is needed to make this reaction move forward. When the reaction is catalyzed by an enzyme, the amount of activation is greatly reduced, making that hump easier for the reactants to get over.
Enzymes are able to lower the activation energy of a chemical reaction by making changes to the transition state of the reaction. By stabilizing the transition state, the reaction will move toward the transition state more easily. Without an enzyme, the transition state is often not energetically favorable. The enzyme will alter the transition state in order to make it more favorable and to move the reaction forward. Similarly, the enzyme can lower the energy of the transition state, which will allow the reaction to move forward.
Inhibition
Inhibitors bind to an enzyme to decrease its activity. The prevention of substrate-enzyme binding is a form of regulation. Negative feedback is an example of a time when inhibitors are important. If the body has produced too much of the final products of a reaction, those final products can feedback to the reaction and prevent the enzyme and substrate from binding. In essence, in negative feedback, the end products are telling the body to stop creating them.
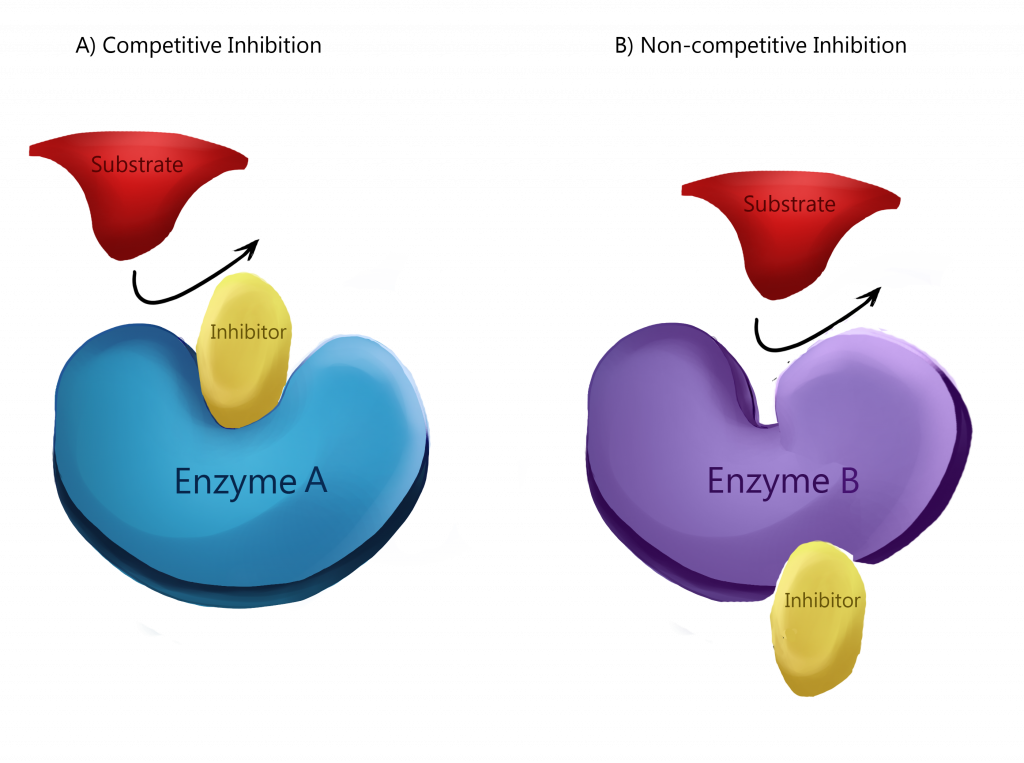
There are two types of inhibition that are used for regulation, competitive and non-competitive. In competitive inhibition, the inhibitor binds directly to the active site, effectively completely blocking access from the substrate.
Non-competitive inhibition, also known as allosteric inhibition, is when the inhibitor binds to a different part of the enzyme but induces a change in the active site to prevent binding by the substrate. The binding often changes the shape or charge of the binding site, preventing the substrate from being able to bind. The other way to inhibit is to bond. These processes all help to regulate rates of enzyme activity.
Factors that Affect Enzyme Activity
Enzyme activity is affected by many factors, including temperature and pH. An increase in temperature increases the rate at which the molecules in a system move. This increase in temperature will allow the substrates and enzymes to locate each other more quickly. However, there is a point at which the enzyme will become denatured due to the higher temperature, adding stress to its bonds. Many enzymes operate at an ideal temperature called the optimum temperature.
pH can also affect an enzymes activity. pH controls the balance between positively and negatively charged amino acids. Ionic interactions are important to hold the enzymes together. Most enzymes have an optimum pH between 6 and 8.
Example
Now that we have covered the topic of enzymes, let’s explore a real life example. At this point in your studies, you may have come across an enzyme called DNA polymerase (if you haven’t please check out AP® Biology Crash Course Review: DNA Replication). DNA polymerase is an enzyme that catalyzes the chemical reaction of deoxynucleoside triphosphate plus DNA to diphosphate and DNA (plus the nucleotide).
In this reaction, the enzyme breaks a phosphate bond from the deoxynucleoside triphosphate and uses that energy to add the nucleotide base to the DNA molecule. Without DNA polymerase, this process would not be able to occur because it is energetically unfavorable to catalyze. If this process could not occur, our cells would not be able to replicate and repair. This would result in death of the organism.
AP® Biology Question
Now that we have reviewed the information you need to know about enzymes for the AP® Biology exam, here is an example of a multiple choice question you could see:
Which of the following is characteristic of enzymes?
A. They lower the energy of activation of a reaction by binding the substrate.
B. They raise the energy of activation of a reaction by binding the substrate.
C. They lower the amount of energy present in the substrate.
D. They raise the amount of energy present in the substrate.
What did you pick? If you chose A you are correct! Enzymes lower activation energy when they bind to the substrate and alter the transition state. If you had trouble with this question, go back through and read this review. If you have any questions, let us know in the comment section!
Summary
In nearly every chemical reaction of life, enzymes are used. Dr. Richard Wolfenden recently found that if enzymes were removed, the biological reactions necessary to life would take 2.3 billion years to spontaneously occur. Clearly, enzymes are a necessary part of life!
In this AP® Biology Crash Course Review, we went over the general structure of an enzyme and its activation site. We then reviewed what exactly enzymes do and how they do it. We then reviewed different types of activation and inhibition molecules. Finally, we wrapped up with an example of a real life enzyme and why it is important to survival.
The AP® Bio exam will likely have questions about enzymes on it. Do you feel prepared? Let us know!
Need help preparing for your AP® Biology exam?
Albert has hundreds of AP® Biology practice questions, free response, and full-length practice tests to try out.