The AP® Exam is often a source of trepidation for many high school students. Let’s be honest: three hours of wracking your brain, recalling facts from an entire year’s worth of information isn’t exactly fun. While the AP® Chemistry Exam’s multiple-choice section may not be enjoyable, however, it is certainly doable. That 5 is attainable, and the right approach and resources can help you pass with flying colors. Here we’ve compiled some detailed information to help you become acquainted with the AP® Chemistry multiple-choice questions in the hope of helping you relax, study more efficiently, and score high on test day.
What is the format of the AP® Chemistry Exam?
The exam for an AP® course is perhaps the most important part. If you don’t take the exam, colleges considering giving you AP® credit will have no way of knowing how well you did in the class. Thus, just as earning A’s and B’s on your AP® coursework is important, achieving 4’s and 5’s on your exams is equally vital. When preparing for the AP® Chemistry multiple-choice questions, you will want to familiarize yourself in as much detail as possible with the exam content for the course.
First, know that the AP® Chemistry Exam will strive to test your conceptual, lasting understanding of the topics taught in class through inquiry-based reasoning rather than regurgitating memorized facts or just plugging numbers into a formula. You’ll be asked to explain chemical phenomena using modeling and to justify concepts using mathematical formulas.
Furthermore, a common way to test a student’s conceptual understanding of a science topic is to require experimental design, ranging from designing an experiment to analyzing the data and sources of error. Thus, the AP® Chemistry Exam will involve a combination of memorized facts and their application, so as you study, consistently work not only to learn information but to also understand why it is important and how it fits into the field of chemistry as a whole.
The AP® Chemistry Exam lasts for three hours and fifteen minutes and is divided into two parts.
Section I lasts for 90 minutes and consists of 60 multiple-choice question which will either be discrete or presented in sets. Question sets are comprised of a series of questions based on a data set or other stimulus.
Section II, lasting for 105 minutes, includes three long free-response questions and four short free-response questions. You will be provided with a periodic table and equation list for use throughout the exam and will be allowed to use a scientific calculator only during Section II.
Why is the AP® Chemistry Multiple Choice Section Important?

Often, students can be more intimidated by the free-response portion of an AP® exam and thus spend a disproportionate amount of time preparing for it. Before you get caught in this trap, know that the multiple-choice section of the AP® Chemistry Exam accounts for 50 percent of your overall score, meaning Section I is just as important as Section II. Keep in mind that focusing solely on free-response questions can only earn you a 50 percent, so take the multiple-choice section seriously.
What Content is Covered in the Multiple Choice Section of AP® Chemistry?
A key to winning a game or defeating an enemy is understanding your opponent. Thus, to do well on the multiple-choice section of the AP® Chemistry exam, you should have a basic understanding of which key concepts you will be responsible for learning.
The CollegeBoard provides an extensive course and exam description including a concept outline for AP® Chemistry, which will tell you in great detail what you will be expected to know. There are four big ideas, each of which is divided further into enduring understandings, which are divided even further into points of essential knowledge, science practices, and learning objectives. Any of this is fair game for the exam. To get you started, here are the big ideas covered in the course, as well as their respective enduring understandings:
1. The chemical elements form the building blocks of matter, and all matter can be described by the arrangements of atoms.
- Atoms, molecules, and elements
- The unique atomic structure of each element
- Periodicity, periodic trends, and their connection to electronic structure
- Atomic models
- Conservation of matter
2. Atomic, molecular, and ionic structure, as well as the forces between them, explain the chemical and physical properties of materials.
- Physical properties
- Intermolecular forces
- Chemical bonding
- Connection between bonding and properties
3. Changes in matter involve the reorganization and rearrangement of atoms as well as electron transfer.
- Balanced chemical equations
- Classifying chemical reactions
- Chemical/physical transformations and energy changes
4. Reaction rate is determined by the details of molecular collisions.
- Measuring reaction rate and the factors on which it depends
- Molecular collisions and their connection to elementary reactions
- Series of elementary reactions
- Catalysts and reaction rate
5. The laws of thermodynamics and their usefulness in describing the role of energy and in predicting the direction of changes in matter.
- Heat and energy exchange
- Conservation of energy
- Bond energy
- The energy required to break intermolecular interactions
- Connection between enthalpy, entropy, and physical and chemical processes
6. The formation and breakage of bonds and intermolecular attractions are in dynamic competition, sensitive to initial conditions and external perturbations.
- Chemical equilibrium
- The response of systems at equilibrium to external perturbations
- Connection between chemical equilibrium, acid-base chemistry, and solubility
- Connection between the equilibrium constant, temperature, and Gibbs free energy
How to Prepare for AP® Chemistry’s Multiple Choice

Now that you have a good understanding of the exam content let’s take a look at some specific AP® Chemistry tips useful in preparing for the multiple-choice section.
1. Use lecture and class time to your advantage.
Class time is free test preparation, so use it! Engage in the lecture by taking good notes, asking questions for clarification, and answering questions for practice.
2. Organize your information.
Keep your notes neat so you can eliminate stress and confusion while studying. Make flashcards to aid memorization. Fill gaps in your notes using your textbook.
3. Learn to recognize patterns as well as their exceptions.
Multiple-choice questions often require you to choose the “best” answer or the one “false” answer. Strive to know each detail about a concept and to make connections to other concepts.
4. Learn to read charts, graphs, and data tables.
If you know how to interpret a data set, you’ll have a free answer to a multiple-choice question.
5. Learn to think conceptually.
Analytical, conceptual thinking is a learned art. Work to move beyond mere memorization to relating concepts to each other, to the larger field of chemistry and other scientific disciplines. Listen carefully when your teacher makes these connections, and familiarize yourself with this type of thinking by completing practice questions.
How to Answer AP® Chemistry Multiple Choice Questions.

In addition to having a good handle on the chemistry material itself, work to apply several test-taking strategies while practicing for and taking the multiple-choice section of the AP® Chemistry Exam. Having these procedures in place will maximize your score and reduce stress on test day. Use the following AP® Chemistry tips in answering the multiple-choice questions:
1. Manage your time.
The best way to do this is to mark and skip difficult questions as you move through the test. Answer easy questions for guaranteed points, then return to your marked questions at the end.
2. Understand the question.
Circle words that will determine your answer to the question, such as only, best, or not. If you’re having a difficult time visualizing or recalling a concept, draw a quick sketch or figure. Trying to hold and interact with information just in your head is challenging, so cut yourself as much slack as possible by writing and drawing.
3. Eliminate choices you know to be incorrect.
Try to answer a question before looking at the options. If you aren’t sure, then begin by eliminating obviously wrong answer choices. There’s no penalty for a wrong answer, so you significantly increase the probability of answering correctly by removing one or two options.
What are AP® Chemistry Multiple Choice Questions Like?
To familiarize yourself with the setup of AP® Chemistry multiple-choice questions and the thought process behind determining the answer, peruse the following two examples from the CollegeBoard’s released 1999 AP® Chemistry Exam:
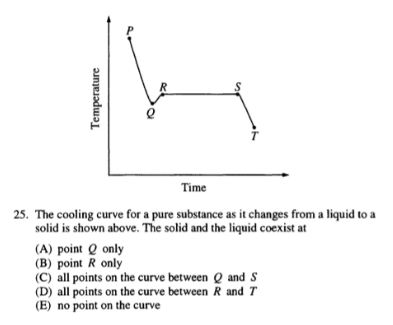
When presented with a graph, begin by reading it to the best of your ability and answering a few questions. How are the axes labeled? What trend is shown? Have I seen something like this before?
Often, important information about the graph will be given in the question, so read this too. Answering this particular question relies on your knowledge of phase changes, knowing that the section of the graph depicting constant temperature corresponds with the coexistence of two phases as one changes into the other. Thus, the correct answer is Choice C.

Questions such as this can be quite tricky. The best approach is to write “true” or “false” next to each statement, then select the corresponding answer choice. This question tests your understanding of ionic solids, lattice energy, and Coulomb’s law. The compound with the higher lattice energy will melt at a higher temperature, and lattice energy is directly related to ionic charge and indirectly related to ionic size. Thus, Statements I and II are correct and relevant. Statement III, though relevant, is incorrect, as the O2- ion is larger than the F– ion. This makes Choice B the correct answer.
These questions should give you an idea of what is involved in the multiple-choice section of the AP® Chemistry Exam. For additional insight and help with multiple-choice questions, you can check out Albert’s AP® Chemistry practice questions.
How can I Practice AP® Chemistry Multiple Choice?
Now that you are armed with information about the AP® Biology multiple-choice questions, it’s time to study and get that 5. The best course of action involves developing your own AP® Chemistry study plan so you can work ahead, paying attention in class, and utilizing excellent resources. Albert’s chemistry resources provide an extensive bank of questions designed with the student in mind. Still struggling to grasp that difficult concept? This blog contains articles to guide you through a variety of AP® Chemistry topics. For further study tips, check out this AP® Chemistry review guide.
Looking for AP® Chemistry practice?
Kickstart your AP® Chemistry prep with Albert. Start your AP® exam prep today.








