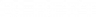Chemistry isn’t just about mixing colorful liquids in beakers and waiting for an explosion—it’s a world of ratios, reactions, and relationships. At the heart of understanding this fascinating world lies the concept of the molar ratio, a key player in predicting the outcomes of chemical reactions. But what exactly is a molar ratio, and why is it so important? Simply put, it quantifies the proportions of reactants and products in a chemical equation, and accounts for every atom according to the law of conservation of mass. Remember learning about balanced equations? It’s time to put that knowledge into action. In this blog post, we’ll dive deep into the world of molar ratios, from what they are to how to find them using balanced equations. Whether you’re a budding chemist or just curious about the science behind the reactions, you’re in the right place to uncover the secrets of how to find the molar ratio.
What We Review
What is Molar Ratio?
Imagine you’re following a recipe to bake a cake. You wouldn’t just throw in random amounts of flour, sugar, and eggs, right? Just like in baking, chemistry requires precise measurements to get the desired outcome. This is where the concept of the molar ratio comes into play, acting as the “recipe” for chemical reactions.

A molar ratio is the proportion of moles of one substance to the moles of another substance in a chemical reaction. The coefficients of the substances in a balanced chemical equation show the molar ratio relationship. For example, in the reaction to produce water (2H_2 + O_2 \rightarrow 2H_2O ), the molar ratio of H_2 to O_2 is 2:1. This means that two moles of hydrogen gas react with one mole of oxygen gas to produce water.
So, why is this important? Chemists predict how much of each reactant they need to produce a certain amount of product without any waste by using molar ratio. It ensures that every atom of the reactants has a place in the products, adhering to the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
Understanding molar ratios is crucial for anyone looking to delve into the world of chemistry, whether it’s synthesizing a new compound in a research lab or figuring out the right amount of baking soda to add to your volcano science project. By mastering this concept, you’ll unlock the ability to navigate through chemical equations with ease, paving the way for exciting experiments and discoveries.
How to Find Molar Ratio
In order to truly grasp the concept of molar ratio, let’s dive into some example problems that highlight how to use this tool in real chemical equations. Curious about how to find the molar ratio? These examples will show you step-by-step how to calculate molar ratios and apply them to predict the amounts of reactants and products in a chemical reaction.
Example 1: Combustion of Propane
The combustion of propane (C_3H_8) in oxygen (O_2) is a common reaction that produces carbon dioxide (CO_2) and water (H_2O). The balanced chemical equation for this reaction is:
C_3H_8 + 5O_2 \rightarrow 3CO_2 + 4H_2O
Question: What is the molar ratio of O_2 to CO_2 in the combustion of propane?
Solution: To find molar ratio, look at the coefficients in your balanced equation. By looking at the balanced equation, we can see that 5 moles of O_2 produce 3 moles of CO_2. Therefore, the molar ratio of O_2 to CO_2 is 5:3.
Example 2: Formation of Ammonia
The Haber process combines nitrogen (N_2) and hydrogen (H_2) gas to form ammonia (NH_3), a crucial component in fertilizers. The balanced equation for this reaction is:
N_2 + 3H_2 \rightarrow 2NH_3
Question: If you start with 4 moles of N_2 how many moles of H_2 are needed to react completely based on the molar ratio?
Solution: From the balanced equation, the molar ratio of N_2 to H_2 is 1:3. This means for every mole of N_2, they require 3 moles of H_2 to react. For 4 moles of N_2, we need:
4\text{ moles of }N_2 \times \frac{3\text{ moles of }H_2 }{1 \text{ mole of }N_2} = 12 \text{ moles of }H_2
Therefore, 12 moles of H_2 completely react with 4 moles of N_2.
Practice Problems: Finding and Applying Molar Ratio
Now that you’ve seen how to find molar ratio and how to use them through example problems, it’s your turn to try solving some on your own. These practice problems will test your understanding of molar ratios and how to apply them to different chemical reactions. Work through these on your own, then scroll down for solutions.
Problem 1: Synthesis of Water
When hydrogen gas (H_2) reacts with oxygen gas (O_2) water (H_2O) forms. The balanced equation for this reaction is:
2H_2 + O_2 \rightarrow 2H_2O
If you have 6 moles of H_2, how many moles of O_2 are needed to react completely, and how many moles of H_2O will be produced?
Problem 2: Decomposition of Potassium Chlorate
Potassium chlorate (KClO_3) decomposes upon heating to produce potassium chloride (KCl). The balanced equation for this reaction is:
2KClO_3 \rightarrow 2KCl + 3O_2
How many moles of O_2 can be produced from the decomposition of 4 moles of KClO_3?
Problem 3: Combustion of Ethanol
Ethanol (C_2H_5OH) combusts in oxygen to produce carbon dioxide and water. The balanced chemical equation is:
C_2H_5OH + 3O_2 \rightarrow 2CO_2 + 3H_2O
If 2 moles of C_2H_5OH are combusted, how many moles of O_2 are required, and what amounts of CO_2 and H_2O are produced?
Problem 4: Production of Ammonium Nitrate
Ammonium nitrate (NH_4NH_3)is produced by the reaction of ammonia (NH_4) with nitric acid (HNO_3). The balanced chemical equation for this reaction is:
NH_3 + HNO_3 \rightarrow NH_4NO_3
If a fertilizer company needs 5 moles of ammonium nitrate, how many moles of ammonia and nitric acid are required to achieve this?
Problem 5: Synthesis of Magnesium Oxide
Magnesium (Mg) reacts with oxygen (O_2) to form magnesium oxide (MgO). The balanced equation for this reaction is:
2Mg + O_2 \rightarrow 2MgO
During a lab experiment, a student reacts 6 moles of magnesium with excess oxygen. How many moles of magnesium oxide does the reaction produce, and how many moles of oxygen are consumed in the reaction?
Tips for Solving:
- Start by identifying the molar ratios between the reactants and products from the balanced chemical equations.
- Use the molar ratios to calculate the amounts of reactants or products as needed.
- Remember to check your work and ensure that the law of conservation of mass is satisfied in your calculations.
Molar Ratio Practice Problem Solutions
Are you ready to see how you did? Review below to see the solutions for the molar ratio practice problems.
Problem 1: Synthesis of Water
When hydrogen gas (H_2) reacts with oxygen gas (O_2) water (H_2O) forms. The balanced equation for this reaction is:
2H_2 + O_2 \rightarrow 2H_2O
If you have 6 moles of H_2, how many moles of O_2 are needed to react completely, and how many moles of H_2O will be produced?
Remember, to find molar ratio, use the coefficients in the balanced equation. The molar ratio of H_2 to O_2 is 2:1, meaning 2 moles of H_2 react with 1 mole of O_2. You will use this ratio to determine how many moles of O_2 are needed to react completely.
6 \text{ moles of }H_2 \times \frac{1 \text{ mole of }O_2}{2 \text{ moles of }H_2} = 3 \text{ moles of }O_2
The molar ratio of H_2 to H_2O is 2:2, which can be simplified to 1:1. Therefore, if 6 moles of H_2 reacts, it produces 6 moles of H_2O.
Problem 2: Decomposition of Potassium Chlorate
Potassium chlorate (KClO_3) decomposes upon heating to produce potassium chloride (KCl). The balanced equation for this reaction is:
2KClO_3 \rightarrow 2KCl + 3O_2
How many moles of O_2 can be produced from the decomposition of 4 moles of KClO_3?
The molar ratio of KClO_3 to O_2 is 2:3. Hence, 2 moles of KClO_3 react with 3 moles of O_2. You will use this to determine how many moles of O_2 will be produced.
4 \text{ moles of }KClO_3 \times \frac{3 \text{ mole of }O_2}{2 \text{ moles of }KClO_3} = 6 \text{ moles of }O_2
Problem 3: Combustion of Ethanol
Ethanol (C_2H_5OH) combusts in oxygen to produce carbon dioxide and water. The balanced chemical equation is:
C_2H_5OH + 3O_2 \rightarrow 2CO_2 + 3H_2O
If 2 moles of C_2H_5OH are combusted, how many moles of O_2 are required, and what amounts of CO_2 and H_2O are produced?
This question has three different parts. The first is determining how many moles of O_2 are needed to react completely with 2 moles of C_2H_5OH. Initially, you must find the molar ratio. The ratio of O_2 to C_2H_5OH is 3:1.
2 \text{ moles of }C_2H_5OH \times \frac{3 \text{ mole of }O_2}{1 \text{ mole of }C_2H_5OH} = 6 \text{ moles of }O_2
The second part asks how much CO_2 is produced from 2 moles of C_2H_5OH. The ratio of CO_2 to C_2H_5OH is 2:1.
2 \text{ moles of }C_2H_5OH \times \frac{2 \text{ mole of }CO_2}{1 \text{ mole of }C_2H_5OH} = 4 \text{ moles of }O_2
The third part asks how much H_2O the reaction produces from 2 moles of C_2H_5OH. The ratio of H_2O to C_2H_5OH is 3:1.
2 \text{ moles of }C_2H_5OH \times \frac{3 \text{ mole of }H_2O}{1 \text{ mole of }C_2H_5OH} = 6 \text{ moles of }H_2O
Problem 4: Production of Ammonium Nitrate
Ammonium nitrate (NH_4NH_3)is produced by the reaction of ammonia (NH_4) with nitric acid (HNO_3). The balanced chemical equation for this reaction is:
NH_3 + HNO_3 \rightarrow NH_4NO_3
If a fertilizer company needs to produce 5 moles of ammonium nitrate, how many moles of ammonia and nitric acid are required to achieve this?
This is a two-part problem, but solved the same way. This is because the balanced equation has a molar ratio of 1:1 for all reactants and products in the equation. Therefore, however many moles you put into the reaction, produces the same amount of moles as products. Therefore, if we want to produce 5 moles of NH_4NH_3, we would need to put in 5 moles of NH_3 and 5 moles of HNO_3.
Problem 5: Synthesis of Magnesium Oxide
Magnesium (Mg) reacts with oxygen (O_2) to form magnesium oxide (MgO). The balanced equation for this reaction is:
2Mg + O_2 \rightarrow 2MgO
During a lab experiment, a student reacts 6 moles of magnesium with excess oxygen. How many moles of magnesium oxide will be produced, and how many moles of oxygen are consumed in the reaction?
This is a two-part problem. The first part asks how many moles of Mg the reaction makes if 6 moles of Mg reacts. The molar ratio of MgO to Mg is 2:2, which can be simplified to 1:1. Therefore, if 6 moles of Mg reacts, 6 moles of MgO will be produced.
The second part of the problem asks how much excess O_2 the reaction uses if 6 moles of Mg reacted. The molar ratio of Mg to O_2 is 2:1. We use this to determine our answer.
6 \text{ moles of }Mg \times \frac{1 \text{ mole of }O_2}{2 \text{ moles of }Mg} = 3 \text{ moles of }O_2
Conclusion

Embarking on the journey through the world of chemistry reveals the intricate dance of atoms and molecules, governed by fundamental principles such as the molar ratio. This concept, akin to the precise measurements in a recipe, ensures that chemical reactions proceed smoothly, with each reactant and product playing its part in the grand scheme of matter transformation.
Understanding molar ratios not only demystifies how substances react in specific proportions but also empowers us with the ability to predict the outcomes of these reactions. Whether it’s synthesizing a new compound in the lab, analyzing environmental samples, or simply marveling at the chemical reactions in everyday life, the knowledge of molar ratios serves as a crucial tool in the arsenal of any budding chemist.
In conclusion, we’ve explored how to find molar ratio and tackled practice problems to solidify our understanding. Remember, the beauty of chemistry lies not just in theoretical knowledge but in applying these concepts to solve real-world problems. So, I encourage you to continue exploring, questioning, and experimenting with the fascinating reactions that make up our world.
Chemistry continually challenges and inspires, and with tools like molar ratios, you can uncover the mysteries that lie in molecules and reactions. So, keep your curiosity alive, and let the molar ratio guide you as you journey through the incredible landscape of chemistry.