Introduction
Lipids have gotten a bad reputation over the past few years due to all the hype about fats being bad, but in reality, lipids are much more than just “fat.” They are, in fact, one of the building blocks of life. In this crash course review, we will go over everything you need to know about lipids to not only be prepared for the AP® Biology exam but also to better understand what an important role lipids play in biology as a whole. We’ll start with going over what lipids are in general; then we will look at how the three main types of lipids differ in structure and function; and finally we’ll have some review questions and a quick recap. By the end of this crash course review, you should feel confident enough in your knowledge of what lipids are and why they are important to be able to answer whatever the AP® Bio exam may throw at you.
What are Lipids?
Lipids, like carbohydrates and proteins, are a class of organic compounds. They are hydrocarbon-based macromolecules that are grouped together because of their hydrophobic qualities. This means that all lipids are insoluble in water, which you may have already noticed if you have ever tried to wash butter or oil off of your hands. There are three main families of lipids: fats, phospholipids, and steroids. Let’s look at each of these in a bit more detail.
Fats
Fats are energy storing macromolecules that are made up of two main components: a molecule of glycerol and three fatty acids. Because of this structure, they are sometimes referred to as triglycerides, with the ‘tri-‘ prefix meaning “three.” These fatty acids are long chains made up a hydrocarbon tail with a carboxyl group head.
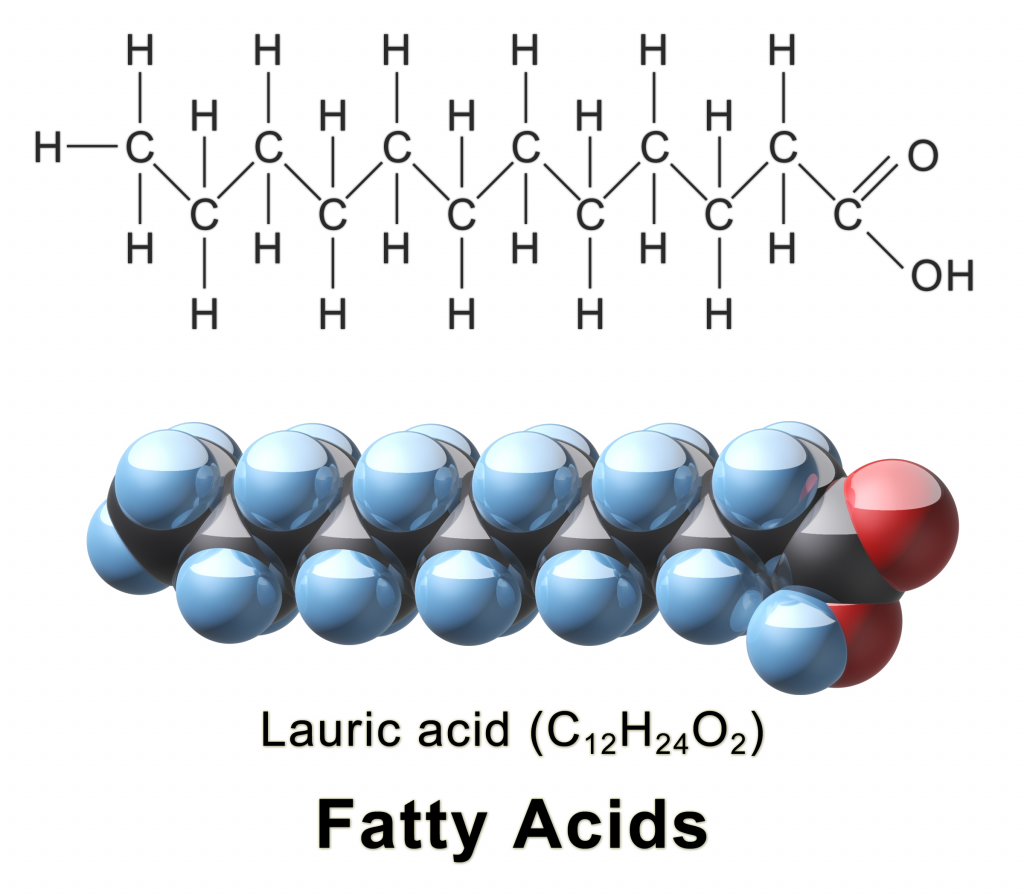 Image Source: Wikimedia Commons
Image Source: Wikimedia Commons
The fatty acids are linked to the glycerol backbone through the process of dehydration synthesis, which you may remember reading about if you have already reviewed other macromolecules like carbohydrates. If not, here is a brief explanation:
Dehydration synthesis, sometimes known simply as condensation, is a process where monomers are bound together through the loss of a water molecule. A covalent bond is formed. The reverse process of dehydration synthesis is hydrolysis.
Now, back to fats. There are two main types of fatty acids: saturated and unsaturated. Saturated fats contain only single bonds between carbon atoms. All the carbons are bonded to hydrogens, and there are no carbon double bonds. Generally, saturated fats come from animals (but also some tropical oils like coconut and palm oil), and they are solid at room temperature. Consumption of saturated fats is linked to heart disease due to plaque deposits in the blood vessels. A good example of saturated fat is butter.
Unsaturated fatty acids have at least one double bond in their chains. This is formed by removing hydrogen atoms from the carbon skeleton, meaning that unsaturated fatty acids have fewer hydrogen atoms than saturated fatty acids (i.e., they are less saturated with hydrogen). Unsaturated fatty acids usually come from plants or fish and are liquid at room temperature. When fats are in liquid form, they are known as oils. Some good examples unsaturated fatty acids include vegetable oils like canola oil and olive oil as well as fish oil.
You may have also heard of a third type of fat called trans fats. Trans fats are sometimes known as ‘partially hydrogenated oils’ because they are created through an industrial process where hydrogen is added to vegetable oils to make them more solid. A good example of this is margarine, which is vegetable oil but can be bought in a solid form similar to butter. Consuming lots of trans fats increases your risk of heart disease, stroke, and type 2 diabetes.
So if fats increase the risks for all these bad things like heart disease and stroke, what are they good for? Fats are incredibly important for energy storage: 1g of lipid will release nine calories when burned, while in comparison 1g of carbohydrates only releases four calories. Although we are lucky enough to live in a time and place where our food sources are abundant, this was not always the case, and certainly still is not the case for most life on earth. Plants and animals need to be able to store energy as fat to be able to access it in times when food is scarce, and their fat reserves are all they can live off of.
Fats also serve the important function of protecting the organs and insulating the body. Whale blubber, for example, is entirely made of fat.
Phospholipids
Phospholipids are very similar to fats in their structure, but instead of having three fatty acids bound to glycerol, they have two fatty acids and a phosphate group (PO4) bound to glycerol.
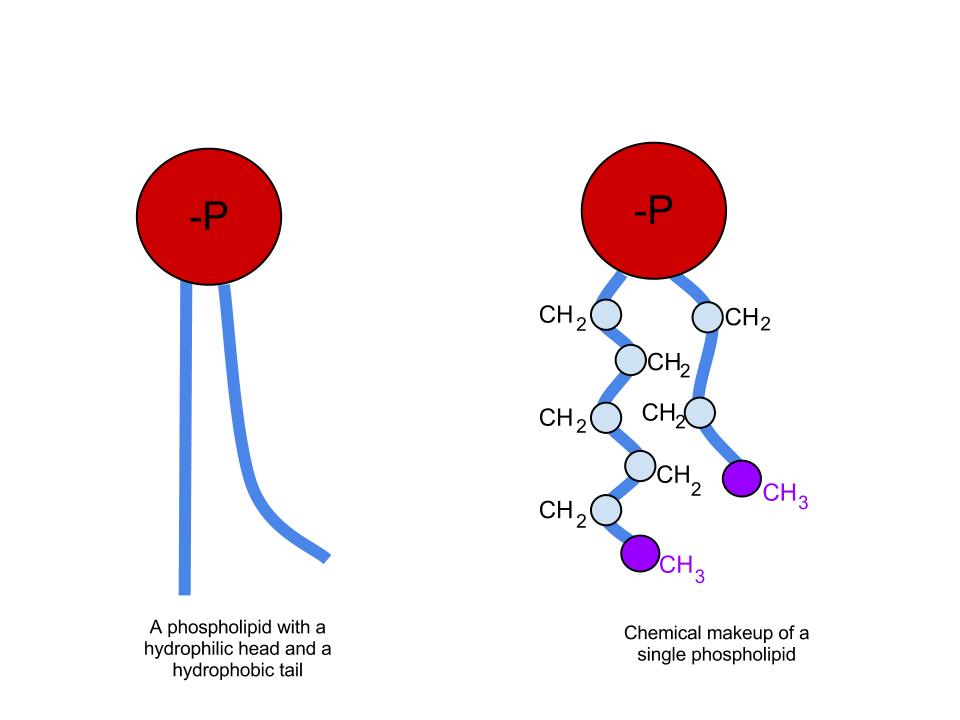 Image Source: Wikimedia Commons
Image Source: Wikimedia Commons
Phospholipids serve essential functions in the structure of cell membranes. While the fatty acid tails are hydrophobic, the PO4 head of a phospholipid is hydrophilic. This allows them to arrange themselves into a phospholipid bilayer, where the hydrophilic heads face outward, and the hydrophobic tails face inward to create a nonpolar zone that is essentially a barrier in water. This is how cell membranes are formed.
Steroids
Steroids are a family of lipids that have quite a different structure compared to fats and phospholipids. Steroids have four fused hydrocarbon rings with various chemical attached to them that determine which specific steroid it is. One steroid you will need to know for AP® Biology is cholesterol. Cholesterol is a component of the plasma membranes in animal cells, making it a vital part of cell structure – it helps keep membranes flexible and fluid. It is also the precursor to many other important steroids, such as the sex hormones testosterone, estradiol, and progesterone.
 Image Source: Wikimedia Commons
Image Source: Wikimedia Commons
Other Lipids
We’ve gone through the three main types of lipids, but there are a few more less-common types that are also worth mentioning. Waxes, for example, are also considered lipids due to their hydrophobic nature. Wax can coat the outside of some plants, as well as the feathers of birds and even the fur of some animals to keep them dry from rain and other water. Omega fatty acids like Omega-3 and Omega-6 are also lipids and are essential for normal growth and brain health. They also protect against cardiovascular disease.
Review Questions
Question 1. Why are sex hormones considered lipids?
A) They consist of fatty acids
B) They are essential to the structure of cell membranes
C) They store energy
D) They are hydrophobic
E) They are hydrophilic
Question 2. What happens when hydrogen is added to vegetable oils?
A) The hydrogenated vegetable oil will have fewer trans fats
B) The hydrogenated vegetable oil will be solid at room temperature
C) The hydrogenated vegetable oil will be less likely to cause heart disease
D) The hydrogenated vegetable oil will become a saturated fat
Question 3. True or False: Of the three main families of lipids, phospholipids are most important for energy storage.
A) True
B) False
Answers
Question 1. The correct choice is option D – they are hydrophobic. The criteria that all lipids must meet to be considered lipids is that they must be insoluble in water.
Question 2. The correct answer is option B – the oil will be solid at room temperature. The process of hydrogenation creates trans fats that cause many health problems.
Question 3. The correct answer is B – False. Of the three main classes, the group of lipids that is most important for energy storage is fats. Phospholipids serve a vital purpose in providing structure for cell membranes.
Crash Course Review Recap
- Lipids are hydrophobic organic compounds that are divided into three main categories: fats, phospholipids, and steroids.
- Fats are composed of a glycerol and three fatty acids and are used for energy storage.
- Saturated fats have single bonds, are solid at room temperature, and generally come from animal sources.
- Unsaturated fats have double bonds, are liquid at room temperature (oils), and generally come from plant sources.
- Trans fats are created industrially by adding hydrogen to vegetable oils.
- Phospholipids have a glycerol, two fatty acids, and a phosphate group; they are essential for the structure of cell membranes.
- Steroids are made of four fused hydrocarbon rings and are important for structural and endocrine functions; main example to know is cholesterol.
Need help preparing for your AP® Biology exam?
Albert has hundreds of AP® Biology practice questions, free response, and full-length practice tests to try out.
Are you a teacher or administrator interested in boosting AP® Biology student outcomes?
Learn more about our school licenses here.








