What We Review
Introduction
Macromolecules are large, complex molecules that form the foundation of life. They are essential in countless biological processes, such as storing energy, building cell structures, and transmitting genetic information. Living organisms rely on these molecules for growth, reproduction, and day-to-day functions.
There are four main types of macromolecules in biology: carbohydrates, proteins, lipids, and nucleic acids. Each category has unique characteristics and roles, yet they work together to keep organisms alive and functioning. Accordingly, this overview explores what macromolecules are, describes each major type, and explains how their composition supports life. These concepts frequently appear in AP® Biology, so understanding them is crucial for success on the exam and in future science studies.
What Are Macromolecules?
The term “macromolecules” refers to large molecules composed of smaller building blocks. The word “macro” means “large,” indicating that these molecules can be massive compared to simpler chemical compounds. A key characteristic of macromolecules is their structure: they often consist of repeating units called monomers, linked together to form polymers.
Macromolecules play essential roles in living organisms. For example, they form cellular structures like cell membranes, serve as catalysts for chemical reactions, and store hereditary information. Since macromolecules support nearly every life function, AP® Biology students benefit from understanding their structure and function.
The Four Major Types of Macromolecules
A. Carbohydrates
Carbohydrates are often referred to as sugars. They provide energy and serve as structural components in plants and some animals.
- Definition and Structure
- Carbohydrates are composed of carbon (C), hydrogen (H), and oxygen (O) in a ratio commonly close to \text{C}_n\text{H}_{2n}\text{O}_n.
- The smallest units of carbohydrates are monosaccharides (simple sugars like glucose).
- Functions in Organisms
- Serve as a primary energy source (e.g., glucose fuels cellular respiration).
- Provide structural support (e.g., cellulose forms plant cell walls).
- Examples
- Glucose is a simple sugar used by cells for immediate energy.
- Starch is stored energy in plants.
- Cellulose provides rigidity in plant cell walls.
Example – Forming a Disaccharide Step-by-Step:
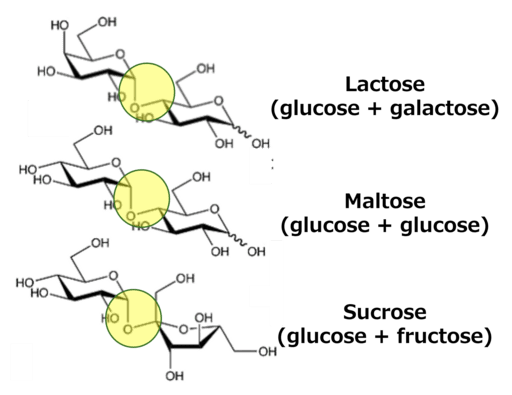
- Start with two glucose molecules (\text{C}_6\text{H}_{12}\text{O}_6).
- During a condensation (dehydration synthesis) reaction, a water molecule \text{H}_2\text{O} is removed.
- The two glucose molecules bond to form maltose (\text{C}_{12}\text{H}_{22}\text{O}_{11}).This process also applies to other sugars that combine to form larger carbohydrates.
B. Proteins
Proteins are vital for numerous biological functions. They are made of amino acids, each containing an amino group, a carboxyl group, and a unique side chain.
- Composition and Structure
- Proteins are polymers built from monomers called amino acids. There are 20 different amino acids in most living organisms. These link together via peptide bonds, creating long chains (polypeptides).
- Functions
- Enzymes that speed up chemical reactions
- Structural support (e.g., in muscle and hair)
- Transport (e.g., hemoglobin carries oxygen in blood)
- Examples
- Hemoglobin helps transport oxygen.
- Enzymes like amylase assist in breaking down starch.
Example – Creating a Dipeptide Step-by-Step:
- Start with two amino acids, each having an amino group (\text{-NH}_2) and a carboxyl group (\text{-COOH}).
- Link the amino group of one amino acid to the carboxyl group of the other.
- A water molecule \text{H}_2\text{O} is released, forming a peptide bond and producing a dipeptide. This reaction can continue to form polypeptides and, eventually, functional proteins.
C. Lipids
Lipids are a broad group of molecules including fats, phospholipids, and steroids. Although not always considered polymers in the same way as proteins or carbohydrates, they are essential in storing energy and forming cell membranes.
- Definition and Types
- Lipids are mostly composed of carbon and hydrogen, with fewer oxygen atoms than carbohydrates. Major types include:
- Fats (triglycerides)
- Phospholipids (make up cell membranes)
- Steroids (cholesterol, hormones)
- Lipids are mostly composed of carbon and hydrogen, with fewer oxygen atoms than carbohydrates. Major types include:
- Functions
- Long-term energy storage (fats)
- Insulation in animals
- Formation of cell membranes (phospholipids)
Example – Creating a Triglyceride Step-by-Step:
- Start with one glycerol molecule (\text{C}_3\text{H}_8\text{O}_3) and three fatty acids.
- Each fatty acid attaches to one of glycerol’s hydroxyl groups (\text{-OH}) through a condensation reaction.
- Three water molecules are released, forming a triglyceride (a common dietary fat).
D. Nucleic Acids
Nucleic acids, such as DNA and RNA, carry genetic instructions for the cell. They play a central role in protein synthesis and inheritance.
- Structure (DNA and RNA)
- Nucleic acids are polymers made of nucleotides. Each nucleotide includes a sugar (deoxyribose in DNA or ribose in RNA), a phosphate group, and a nitrogenous base.
- Functions
- DNA stores genetic information.
- RNA helps convert genetic instructions into proteins during protein synthesis.
- Importance in Protein Synthesis
- During transcription, the cell creates an RNA copy from a DNA template. During translation, this RNA is used to assemble amino acids, forming proteins that carry out various tasks in the organism.
Example – Building a Short DNA Strand Step-by-Step:
- Assemble nucleotides, each with a phosphate group, sugar (\text{C}_5\text{H}_{10}\text{O}_5 in RNA or \text{C}_5\text{H}_{10}\text{O}_4 in DNA), and a base (A, T/U, C, or G).
- Form phosphodiester bonds between each nucleotide.
- Two complementary strands of DNA hydrogen bond together, creating the signature double helix.
Composition of Macromolecules
A. Elements That Make Up Macromolecules
Carbon is central to the structure of macromolecules because it can form up to four covalent bonds, allowing a diverse range of complex shapes. Other essential elements include hydrogen, oxygen, nitrogen, phosphorus, and sometimes sulfur. For instance, amino acids and nucleic acids contain nitrogen, while the phosphate groups of nucleic acids contain phosphorus.
B. Building Blocks of Macromolecules
Monomers are the small molecular units that can be linked together. Polymers form when many monomers join in a chain. Examples include:
- Glucose monomers forming starch (a carbohydrate polymer)
- Amino acids joining to form proteins
- Nucleotides assembling to make nucleic acids
Understanding monomer–polymer relationships is key to grasping how organisms build, break down, and rearrange molecules.
The Role of Macromolecules in Growth and Reproduction
Living organisms must constantly exchange matter with their environment. Nutrients that contain essential building blocks allow organisms to maintain their bodies, repair tissues, and reproduce. Macromolecules play a direct role in these processes because they:
- Provide raw materials for cell structures (phospholipids in cell membranes, proteins in muscle fibers)
- Facilitate chemical reactions (enzymes)
- Store and transfer genetic information (DNA and RNA)
For instance, the body breaks down dietary proteins into individual amino acids and uses them to build new proteins. Similarly, it metabolizes carbohydrates from food to release energy, fueling growth and reproduction.
Examples in Living Organisms
- Plants use carbohydrates produced during photosynthesis to grow leaves and stems.
- Animals ingest proteins to repair muscles and produce enzymes.
- All organisms rely on nucleic acids for cell division and passing on traits.
Conclusion
In summary, macromolecules—carbohydrates, proteins, lipids, and nucleic acids—are essential to life. They form the structural framework of cells, store and transmit genetic information, and facilitate essential biochemical reactions. Understanding these molecules helps explain how organisms grow, reproduce, and maintain homeostasis.
Students preparing for the AP® Biology exam benefit from a detailed knowledge of these molecules and their functions. Investigating how macromolecules interact within the body or in plant cells offers a clear view of the complexity of life. Diving deeper into their concepts allows learners to see biology at both the molecular and organismal levels.
Exploring practice problems, studying reaction mechanisms in detail, and examining real-world examples such as nutrition and genetics can deepen understanding. This knowledge serves as a foundation for more advanced topics in biology and related fields.
Practice Problems
- Write a short paragraph describing how the structure of phospholipids contributes to cell membrane function.
- Compare and contrast the roles of DNA and RNA in protein synthesis.
- Explain how the arrangement of carbon atoms enables carbohydrates to store and release energy efficiently.
Quick Reference Chart
Below is a concise chart highlighting key symbols and words related to macromolecules.
| Key Term | Definition/Role |
| Macromolecule | Large molecule made up of smaller subunits (monomers) |
| Monomer | Basic building block of a polymer |
| Polymer | Chain of many monomers linked together |
| Carbohydrates | Sugars used for energy and structural support; general formula near \text{C}_n\text{H}_{2n}\text{O}_n |
| Proteins | Chains of amino acids serving as enzymes, transport molecules, structural support, and more |
| Lipids | Fats, phospholipids, and steroids; important for energy storage, insulation, and cell membranes |
| Nucleic Acids | DNA and RNA, made of nucleotides, carrying genetic information |
| Amino Acids | Monomers of proteins; contain an amino group and a carboxyl group |
| Nucleotide | Monomer of nucleic acids; consists of a sugar, phosphate group, and nitrogenous base |
| Enzyme | Protein that speeds up chemical reactions |
Sharpen Your Skills for AP® Biology
Are you preparing for the AP® Biology test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world math problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
Need help preparing for your AP® Biology exam?
Albert has hundreds of AP® Biology practice questions, free response, and full-length practice tests to try out.