Are you planning on taking the upcoming AP® Chemistry exam, but worried that your chemistry knowledge has gotten a little flabby around the midsection? Don’t worry, we have just the thing to get your mind in the right kind of form for the exam. Like any good 30-day workout routine designed to get your body in shape, we have created a daily study plan that will help tone your understanding of atomic structure, beef up your knowledge of how chemicals react, and strengthen your comprehension of intermolecular attraction. This AP® study guide is designed to give you everything you need to review and learn how to score a 5 on the AP® Chemistry Exam.
Key Things to Remember While Using this AP® Study Guide
– From The CollegeBoard: “The AP® Chemistry course provides students with a college-level foundation to support future advanced course work in chemistry. Students cultivate their understanding of chemistry through inquiry-based investigations, as they explore topics such as: atomic structure, intermolecular forces and bonding, chemical reactions, kinetics, thermodynamics, and equilibrium.”
– Remember, the requirements for AP® Chemistry include a minimum of 16 hands-on laboratory tests, of which 6 need to be inquiry. Make sure that you have this requirement accommodated before you attempt to take the exam.
– The exam itself consists of 60 multiple choice questions, for which you have 90 minutes to complete. This will make up 50 percent of your exam score. There will also be 7 free response question of varying length that will take up 105 minutes of your allotted time. This will make up the other 50 percent of your exam score.
– Remember, this is just a guide! We are your personal trainer providing the study plan, but if you find that certain recommendations just aren’t working, feel free to move things around. Or add something new to the mix that you know works. We all learn differently—make this AP® study guide work for you.
– Finally, stay healthy! Eating well and getting plenty of rest are absolutely essential to successfully keeping the mind in shape. If you ever feel yourself getting tired following this study plan, get up and do a couple of quick stretches, or go for a short walk. Your brain will appreciate the extra blood flow.
What You Will Need

– Access to Albert.io’s AP® Chemistry homepage. The practice questions, in particular, will be an essential part of our study plan regiment. Albert.io provides the helpful tips that you’ll need to succeed when taking the AP® Chemistry exam.
– A flashcard site such as Quizlet. Alternatively, you can just use regular notecards. Whichever format you like better.
– Note taking materials. These can be digital or good ole’ fashioned pen and paper, whichever you prefer. You will be taking notes daily, so make sure you are comfortable with whichever you choose.
– The CollegeBoard homepage for AP® Chemistry. It will be essential to read through the CollegeBoard’s explanations on how the AP® Chemistry course has been designed and how the exam process will be taking place. Make sure you thoroughly read through the AP® Chemistry information provided by the CollegeBoard’s website.
– Bozeman Science’s AP® Chemistry study guide. These science videos will help you understand the key concepts covered in the AP® Chemistry course.
– Your own AP® Chemistry Textbook, or an online source of equal quality. Sometimes it helps to review this information from another source if you’re hitting a wall with how Albert.io or CollegeBoard describes it. Having a backup will help make sure you can find another approach to learning.
Optional (but helpful) Stuff:
– Any AP-style workbooks or study guides your teacher provides, or any supplemental material you find helps your study of the main materials. It never hurts to pad your options for learning and practicing the material!
– A dictionary, be it print or online. Not all chemistry terms are self-explanatory.
How to Use the Study Plan
– We have designed this AP® study guide to revolve around the specific expectation laid out by the CollegeBoard for the topic of chemistry. The CollegeBoard has identified six “Big Ideas” that students must master. We have developed this routine around these six major themes, providing you with the best opportunity to comprehensively understand the CollegeBoard’s expectations.
– But this also leaves only four to five days for each of these sections, so you must be diligent and keep on top of your work if you want to succeed.
– You should be spending a minimum of one to two hours studying each day. The chemistry exam is known for being difficult, and although this AP® study guide will help you excel at it, it will take work on your part as well.
– But we can’t have you overload, either. It is important that you stay happy and healthy. Don’t overexert yourself. Keep on top of your sleep, healthy eating, and physical fitness. This will ensure that the information you are learning will be retained.
– We have also worked in a study break after the of each Big Idea section so you can both decompress and process all of the information you’ve learned. This will help to ensure that your brain doesn’t get too cluttered when sorting out all of this information in such a short period of time.
Let’s Get Started!

The chemical elements are fundamental building materials of matter, and all matter can be understood in terms of arrangements of atoms. These atoms retain their identity in chemical reactions.
Day 1
– First and foremost, we need to familiarize ourselves with the CollegeBoard’s expectations and policies. Read through the AP® Chemistry Course Overview and get acquainted with the structure of the course, exam expectations, and example questions.
– It is important that you take notes on the information you are gathering. Jot down key concepts that you think might help you in the future, but, in particular, write down where you are struggling. What do you need to work on the most? These are the things you are going to want to focus on throughout this 30-day study guide.
– Read through pages 1 through 7 of the CollegeBoard’s AP® Chemistry Course and Exam Description, paying particular attention to the ways that the “Big Idea” framework applies to the course expectations.
– Familiarize yourself with Albert.io’s AP® Study Guide pages, getting a feel for how our website works and where all of the information is located.
– Albert.io also has an excellent study guide, The Ultimate Study Guide to AP® Chemistry, which you should familiarize yourself with. This guide will become your best friend during this 30-day period.
Now, let’s get into the nitty-gritty of this study plan:
– Read through pages 8-12 of the “Big Idea 1” chapter in the CollegeBoard’s AP® Chemistry Course and Exam Description. Here you’ll familiarize yourself with the introductory material for the course.
– Watch the videos entitled “Molecules and Elements” and Chemical Analysis” from Bozeman Science’s AP® Chemistry
– For each and every day you are reading material or watching a video, you will want to make somewhere between 10 and 20 flashcards (either with note cards, a folded piece of paper, or online on a site such as Quizlet). Choose a term of importance, writing it down on one side of the card, while putting the definition and a helpful hint down on the other side.
For Example:
(Side A) Solution
(Side B) A homogeneous mixture made up of two or more substances that do not chemically combine; instead, the substances mix uniformly in the solution.
Helpful Hint: Think of it as comparing a cup of (dissolved) sugar water and a cup of water with Lego blocks in it. The solute is the substance dissolved in the solution, and the solvent is the substance doing the dissolving.
– Sometimes it helps to leave some room in the significance section, so you can add more info as you learn.
– Now you are ready to tackle some example questions from Albert.io. Complete all of the questions provided for you in the Atoms and Elements section. These correspond to Enduring Understanding 1.A (EU 1.A).
– Albert.io will provide you with helpful tips and places where you can do better. Take note of your weak spots and write them down in your notebook. These are the places you will have to spend more time on as you progress forward in the days ahead.
– Don’t worry if you did poorly on the example questions, this is Day 1! It’s ok, we are here to help. We will strengthen your Chemistry chops, don’t worry. If you’re already doing really well, great job! But you can always improve, stay on top of this 30-day routine no matter what level you are at.
– Make sure to get some rest so you will be ready for day 2. Onward!
Day 2
– Since we are already familiar with the basics, we are going to dive right into the material. As the days progress, this should start to become routine. Make sure you stay on top of your work and if you start having trouble finding the desire to continue, get a friend in on the action. You can both keep each other in line and keep the study plan going.
– Read through pages 14-16 of the “Big Idea 1” chapter in the CollegeBoard’s AP® Chemistry Course and Exam Description. Make sure that you look up any words that you are not familiar with in a dictionary.
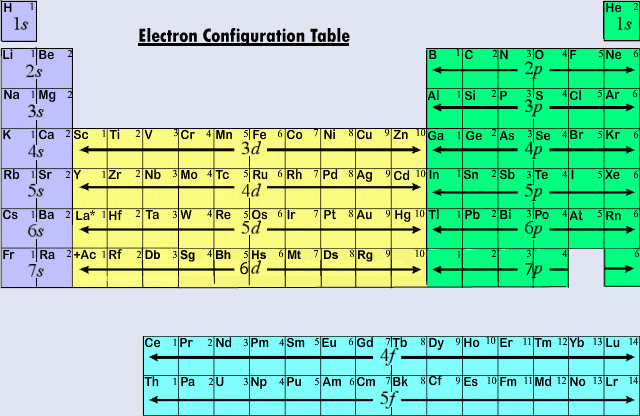
– Watch the videos entitled “The Mole,” “Coulomb’s Law,” and “Electron Configuration” from Bozeman Science’s AP® Chemistry
– Keep on top of your flashcard terms. Make notes on a minimum of ten concepts and/or ideas, but the more the better.
– Complete all of the questions from both Electronic Structure of Atoms and Periodicity (EU 1.B and EU 1.C). If you can, try not to look at any notes and treat this as if you were taking the real test itself.
Day 3
– Read pages 17-18 of the “Big Idea 1” chapter in the CollegeBoard’s AP® Chemistry Course and Exam Description.
– Watch the videos entitled “Periodicity,” “Quantum Mechanical Model,” “Atomic Models,” and “Mass Spectrometry” from Bozeman Science’s AP® Chemistry
– Keep on top of your flashcard terms and your note taking.
– Complete all of the questions from Atomic Models (EU 1.D) If you can, try not to look at any notes and treat this as if you were taking the real test itself. But, at this point, we don’t mind if you peek.
– You’re doing great!
Day 4
– It’s time to finish off the CollegeBoard’s readings on the first Big Idea for this AP® Chemistry. Finish page 19 from the AP® Chemistry Course and Exam Description.
– Take a look at the videos “Light and Matter,” “Symbolic Representations,” and “Conservation of Atoms” from Bozeman Science’s AP® Chemistry Don’t rely solely on these video clips. Make sure that you are watching these alongside the textbook or another online resource that you have picked up for this course. Using these things side by side will improve your ability to better retain what is being said and taught.
– Read the Albert.io article Coulomb’s Law Review.
– Complete all of the questions from Law of Conservation of Mass (EU 1.E). Again, notes are alright but don’t rely on them.
– Today, we are finishing up Big Idea 1, so go over everything covered thus far. Are you still struggling over certain concepts? If so, go over them again.
Day 5
– Ok, you need a little break. Take a long walk, preferably an hour.
– On the walk go over everything you covered this week. Let the ideas flow. Try to remember what you can in your own words.
– Also, bring your notebook and a pen, so if you get stuck on an idea or can’t remember the definition of a term, write it down.
– When you return from your walk, look up the material you couldn’t remember on your walk. Spend the next hour skimming all of your notes—nothing too serious.
– If you can wrangle somebody like a family member or a friend, try to explain everything you’ve learned this week in your own terms to them. Ask them if your explanations were clear. Also have them ask you questions of their own. This will help you understand the concepts in your own way.
– Get a good night’s sleep; we begin a new Big Idea 2 tomorrow.
Start your AP® Chemistry Prep today

Chemical and physical properties of materials can be explained by the structure and the arrangement of atoms, ions, or molecules and the forces between them.
Day 6
– Before we begin by reading through pages 20-24 of the “Big Idea 2” chapter in the CollegeBoard’s AP® Chemistry Course and Exam Description, you are going to want to try and outline all of the concepts that were covered in Big Idea 1. Try to recreate an outline that covers the main themes and concepts of that section of the course. Make sure that you are connecting your ideas together, showing that you have a solid understanding of how atomic structure operates, especially in relation to matter.
– Keep up with the videos from Bozeman Science’s AP® Chemistry. “Solids and Liquids,” “Gasses,” “Solutions,” and “London Dispersion Forces” will be covered in today’s session.
– Make sure that you are taking note of where you are struggling, so you can come back to it later. Continue writing key concepts on your flashcards, but also keep track of what you find confusing in your notebook. These will prove useful for when it comes time to review.
– Complete all of the questions from Macroscopic Properties of Matter (EU 2..A). Now that we are on the second section, eliminate your note references. You should start treating these practice tests more like the real deal.
– You are now ready to tackle examples from the Free Response section provided by Albert.io. This is where you will want to spend a bit of time sharpening your skills. When you answer these questions, take into consideration your grammar, sentence structure, and double-check everything. Start with the Gas Equilibrium FRQ and get a feel for how these are structured. It’s ok to take your time today, but as the month goes by, you should become more and more comfortable with how these work.
Day 7
– Read through pages 24-27 of the “Big Idea 2” chapter in the CollegeBoard’s AP® Chemistry Course and Exam Description.
– Watch the videos entitled “Dipole Forces,” “Intermolecular Forces,” “Covalent Bonding,” and “Ionic Bonding” from Bozeman Science’s AP® Chemistry
– Go over the notes and flashcards you have created thus far. Quiz yourself on how well you have memorized these concepts.
– Work on the examples provided by Albert.io in Intermolecular Interactions (EU 1.B). Make sure that you pay attention to the notes that Albert.io is providing you. These are here to keep you on track, making sure that you focus on your weak points so you can better understand how to improve.
Day 8
– Keep on top of your AP® Chemistry Course and Exam Description and cover pages 28-32 of the “Big Idea 2” chapter. In your notebook, write a two to four sentence description of what you have read thus far in this chapter. Can you clearly lay out the main concepts and themes in a couple of short sentences? If not, keep working on this exercise every couple of days. You should be able to accomplish this for every Big Idea by the end of this study guide.
– Take a look at the videos entitled “Metallic Bonding,” Lewis Diagrams,” and “Ionic Solids” from Bozeman Science’s AP® Chemistry
– Complete all of the questions from Chemical Bonds and Bonds and Properties of Solids (EU 2.C and EU 2.D). Take note of any similarities between these two sections. If you are seeing any connections between the answer you are succeeding with or not doing so well on, take note of these connections.
– Work on the FRQ entitled Localized Electron Bonding: Properties of Molecular Geometry. At this point, you are going to want to start timing yourself. If you do not have a timer or stopwatch app on your phone/computer, you can access one here: online-stopwach.com. Remember you will have 105 minutes to complete 7 of these types of questions, so you should aim for about 15 minutes per question. It’s ok if it takes you a little longer at this point, but try to pay attention to the amount of time you are taking as you progress.
Day 9
– Read through pages 33-38 of the “Big Idea 2” chapter in the CollegeBoard’s AP® Chemistry Course and Exam Description.
– When watching the videos “Metallic Solids,” “Covalent Network Solids,” and “Molecular Solids” from Bozeman Science’s AP® Chemistry page today, write down three key words or concepts from each video. Make sure you put these and their definitions into your growing flashcard pile.
– Read the Albert.io article How to Calculate and Empirical Formula. Make note of the key concepts covered in this article. Add any ideas that you think are important to your flashcard list.
– Tackle the FRQ entitled Metallic Bonding: Using a Model to Explain Metalic Properties. Make sure you pay attention to the “Grading Rubric” provided by Albert.io. These will help you understand how to approach these questions. Try to keep these rubrics in mind as you move forward.
Day 10
– Congratulations, you’ve made it to another day of rest.
– Take your walk, clear your head. Do the same as last time: think about all of the concepts you’ve gone over up to this point.
– Try to make notes on where you are struggling the most. You can work on these later.
– When you get back from your walk. Go over your flashcards once more; keep mental and/or physical notes on where you need the most work.
Start your AP® Chemistry Prep today

Changes in matter involve the rearrangement and/or reorganization of atoms and/or the transfer of electrons.
Day 11
– Before covering pages 39-41 of the “Big Idea 3” section in the AP® Chemistry Course and Exam Description, work on creating another outline for Big Idea 3.
– Watch the videos “Molecular, Ionic, Net Ionic Equations,” Stoichiometry,” and “Synthesis, Decompositions Reactions” from Bozeman Science’s AP® Chemistry
– Keep working on your note taking and the flashcards you are making. You should be seeing a healthy pile of these accumulating.
– Complete all of the questions from Stoichiometry. (EU 3.A) Review your notes and textbook on any areas you got wrong, and try to figure out why you answered the question wrong. Are you missing an important piece of knowledge about the course material, or did you just get tripped up on the wording of the question?
– Get to work on FRQ Stoichiometry And Enthalpy in Chemical Reactions. Remember to keep timing yourself. Also, keep on top of looking at the recommendations provided by Albert.io; these will help to make sure you are improving your FRQ skills.
Day 12
– Once again, it’s time to take a look at the AP® Chemistry Course and Exam Description. Today we are going to cover pages 42-43 of the “Big Idea 3” chapter
– Watch the videos entitled “Neutralization Reactions” and “Redox Reactions” from Bozeman Science’s AP® Chemistry
– Don’t forget to keep on top of your flashcard terms and your note taking.
– Now that you are familiar with the example question format, try to answer the Reactions (3.B) questions to your best ability. Try to space out what level of difficulty and what sections you choose for these questions for.
– Select and work on FRQ Stoichiometry and Calorimetry.
– Today you will also be taking another practice exam. Select the exam from the year 2014. Act as though this is the real deal—no notes and pay attention to time!
Day 13
– Today we are going to finish off the Big Idea 3 chapter, covering pages 44-46 in the AP® Chemistry Course and Exam Description. If there is anything you are having trouble understanding in this chapter write them down. Take a look at these comments as you read through your textbook and watch today’s video—which is going to be “Chemical Change Elements” from Bozeman Science’s AP® Chemistry page—and use these resources to clarify anything you might not be fully grasping. .
– Review all of your flashcards up to this point. Give yourself a little test by randomly selecting 30 cards and see if you can get all of the definitions correct.

– Complete all of the questions from Chemical and Physical Transformations (3.C). Read the explanations for the answers and take notes on the explanations of questions you got wrong.
– Choose FRQ Empirical Formula of a Hydrate, and answer it to your best ability. It may be a good idea to begin repeating the Free Response answers out loud to yourself. Sometimes, what looks good on paper comes out awkward when read out loud. Do this and check for any odd statements or awkward claims.
Day 14
– Watch the videos entitled “Endothermic and Exothermic Reactions” and “Electrochemistry” from Bozeman Science’s AP® Chemistry
– Go through the notes you’ve put in your notebook. Pinpoint where you are struggling the most and focus on them for a minimum of thirty minutes.
– Now it’s time to take a glance at How to Solve Stoichiometry Problems. While reading the article, if you have a way to highlight the key concepts, do so with those you come across. If not, write these main ideas and write them down in your notebook, explaining why they are important and how they connect to other ideas from the course.
– Since, you are right around the halfway mark, now is a good time to go over everything we have covered up to this point. Go over the notes that you’ve taken and make sure you are grasping everything. In particular, review the notes that Albert.io has provided from your test scores. Find your weakest points and work on making them stronger.
Day 15
– One more Big Idea down. Well done!
– Like the other Big Section breaks, try to review everything you’ve learned. But make sure you don’t structure it too much. Let the ideas flow.
– Get a good night’s sleep; we begin a new Big Idea tomorrow.
Start your AP® Chemistry Prep today

Rates of chemical reactions are determined by details of the molecular collisions.
Day 16
– Before we read through pages 47-48 of the “Big Idea 4” chapter in the CollegeBoard’s AP® Chemistry Course and Exam Description, you are going to write a short couple of sentences on what was covered in the last Big Idea section. After this, try to speculate on what will be covered in this section and how you think it will relate.
– Watch the videos entitled “The Rate of Reactions,” “The Rate Law,” and “The Rate Constant” from Bozeman Science’s AP® Chemistry
– After you have finished answering the example questions from Changes in Reactions Rates and Collisions in Reactions (EU 4.A and EU 4.B), create some flashcards based on the main themes coming from these questions.
– Select and work on FRQ Activation Energy. It is now time to have someone else take a look at what you’ve written. Begin with a friend or family member and ask them to take a look at your response. Ask them to proofread and to give recommendations on how you could improve.
Day 17
– Today we are going to finish up the CollegeBoard’s AP® Chemistry Course and Exam Description on Big Idea #4, which means covering pages 49-53.
– Watch the videos entitled “Elementary Reactions,” “Activation Energy,” and “The Reaction Path” from Bozeman Science’s AP® Chemistry
– Review your flashcards once more.
– Tackle the FRQ Gas Equilibrium. After you have finished with this question, write down a short explanation in your notes, how this question relates to other concepts covered in the course.
Day 18
– Watch the videos entitled “Multistep Reactions” and “The Late-Timing Step” from Bozeman Science’s AP® Chemistry
– Complete all of the questions from Elementary Reactions (4.C).
– Choose the FRQ Experimental Determination of Reaction Rates and answer them to your best ability. Now you should have your teachers reading through your responses. Try and get specific feedback on how to improve and where your concepts are the weakest.
Day 19
– Watch the videos entitled “Reaction Intermediates,” “Catalysts,” “and Catalyst Classes” from Bozeman Science’s AP® Chemistry
– Glance over your notebook, dedicating at least twenty minutes to the areas that you are having the most trouble.
– Take out your notebook and read through the article How to Balance Redox Reactions. In your notes, you should try to outline the key concepts of this article. What is the purpose? What central questions is it raising and answering? How does it connect to the concepts we have covered up until this point in our study plan?
– Now, work on the example questions from Catalysts. Are you struggling with a particular type of problem? If so, review your textbook, the informational videos, and the information provided by this guide to better understand how to work on these types of problems.
Day 20
– Yes! The end of another Big Idea!
– After you’ve taken your walk (or whatever healthy activity that helps you relax), sit a friend or family member down and tell them everything you’ve learned this week. Encourage them to ask questions. The more the better. The more you are able to explain these concepts with your own thoughts and without notes, the better grasp you’ll have.
– Get a good night’s sleep; we begin a new Big Idea tomorrow.
Start your AP® Chemistry Prep today

The laws of thermodynamics describe the essential role of energy and explain and predict the direction of changes in matter.
Day 21
– Watch the videos entitled “Temperature,” “Heat Exchange,” “Energy Transfer,” and “Conservation of Energy” from Bozeman Science’s AP® Chemistry
– Don’t slack on your flashcards and note taking now. You’ve come so far!
– Complete all of the questions from Heat Energy and Energy Conservation. (EU 5.A and EU 5.B).
– Work on the FRQ Patterns in Combustion Enthalpies. Make sure you are keeping on track with the timing on these questions.
Day 22
– Before we take a look at the videos “Energy Saving Processes,” “Calorimetry,” “Bond Length and Bond Energy,” and “Enthalpy of Reaction” from Bozeman Science’s AP® Chemistry page, you should create a list of things you would change about how these videos are presented. What would make them better? Try to think of ways you can add information into your studying that will help to make these videos better.
– Now would be a good time to catch up any example questions that you may have missed or skipped. Make sure that you are all caught up by this point.
– Work on the FRQ Calorimetry. Hopefully your friends and family will be tired of hearing about your FRQs at this point—keep looking to them for feedback.
Day 23
– Watch the videos entitled “Intermolecular Potential Energy,” “Chemical and Physical Processes,” “Biological and Polymer Systems,” and “Entropy” from Bozeman Science’s AP® Chemistry
– Keep reviewing your flashcards and notes.
– Work on the example questions from Bond Energy and Intermolecular Force Energy (EU 5.C and EU 5.D). If at this point, you’ve had to skip a few questions for whatever reason, make sure to take questions you haven’t answered yet.
– Get to work on the FRQ Molecules: Propane and N-propanol. After you have finished with the example question, write and explanation of how you came to the answer. Try to tell an imaginary reader how you came to your response, why you chose the methods you used, and why it’s important to ask this kind of question.
Day 24
– You may have noticed that you have not been asked to read through the CollegeBoard’s AP® Chemistry Course and Exam Description for this Big Idea yet. That’s because we would like you to create an outline of what you have covered thus far in your textbook and the videos you’ve been watching. Highlight and write down the key concepts and themes. Put in as much detail as you can. Now read the CollegeBoard’s Big Idea 6 information (pages 54-68). How does this information compare to the outline that you’ve created? If they are drastically different, you will need to review the information give to you for the week.
– Watch the videos entitled “Spontaneous Processes,” “Using Gibbs Free Energy,” “Driving Nonspontaneous Processes,” and “Kinetic Reaction Control” from Bozeman Science’s AP® Chemistry
– Go ahead and take a look at the Albert.io article on The Krebs Cycle. In your notebook, answer the question in a few short statements: How do the concepts covered in the article connect to what we are covering right now and what we have covered up to this point?
– Go to Albert.io and work on questions from Enthalpy and Entropy. It may seem repetitive to keep working on these example questions, but make sure you keep up with them. Doing this is going to keep you familiar with not only concepts but with the format of the testing.
– Today you will also be taking another practice exam. Select the exam from the year 2015. Act as though this is the real deal—no notes and make sure to pay attention to time!
Day 25
– You’ve made it to the final Big Idea! Congratulations!
– This is probably the most critical period. At this point, you might be feeling a little burned out. But stay with us, we’ll get you through this.
– On top of staying healthy with a walk this week, check in with your general health. Make sure that you are getting enough sleep, eating the right kind of food, and staying on top of your physical activities.
– Keeping your mind and body in shape this week is almost just as important as the studying you will be doing. Stay on top of all of this.
– You’ve made it to the home stretch! Onward!
Start your AP® Chemistry Prep today

Any bond or intermolecular attraction that can be formed can be broken. These two processes are in a dynamic competition, sensitive to initial conditions and external perturbations.
Day 26
– Instead of beginning with the CollegeBoard’s AP® Chemistry Course and Exam Description, you should try and create an outline for all of the previous Big Ideas covered thus far in this study guide. Try to be as clear and specific as possible. If you need to relook over some of your notes, it is ok, but you should be able to do this with confidence.
– Take a look at the videos “Reversible Reactions” and “The Reaction Quotient” from Bozeman Science’s AP® Chemistry Try to outline each of these videos in your notes, taking special care to find points of connection between them.
– Keep reviewing your flashcards.
– Keep working on your example questions; today we are turning to those from Reversible Reactions (6.A).
– Get to work on the FRQ Gas Equilibrium. If you are having more difficulty with the FRQs, you may need to work on more example questions. Visit the CollegeBoard website for more examples so you can focus on these.
Day 27
– Read through pages 69-80 of the “Big Idea 6” chapter in the CollegeBoard’s AP® Chemistry Course and Exam Description.
– Watch the videos entitled “Equilibrium,” “The Equilibrium Constant,” and “LeChatelier’s Principle” from Bozeman Science’s AP® Chemistry
– Don’t slack now, keep up with your note taking.
– It is now time to complete the Albert.io example questions. Complete those from Le Chatlier’s Principle and Acid-Base and Solubility Equilibria (EU 6.B and EU 6.C). Also, if you have not completed any other questions, now is the time to do so. Make sure you finish these, keeping notes on where you are struggling and where you are succeeding.
Day 28
– It’s time to finish up with the “Big Idea 6” chapter in the CollegeBoard’s AP® Chemistry Course and Exam Description. Now make your final Big Idea outline for #6. Cover the main concepts covered, using your textbook to supplement and add details to the information you are writing down.
– Watch the videos entitled “Equilibrium Disturbances” and “Acid Based Equilibrium” from Bozeman Science’s AP® Chemistry
– Select, at random, thirty example questions provided by Albert.io. You will have already completed these questions, but you should do so one more time. If you find yourself stumbling on any of these, make sure you review your notes and textbook in order to help you understand what you are doing wrong.
– Review all of the FRQ you have answered. Ask yourself: Which ones were the strongest? Weakest? Can you see yourself getting progressively better? Take notes in your notebook on where you need to do better. Be as specific as possible.
Day 29
– Read through the rest of the material provided by the CollegeBoard’s AP® Chemistry Course and Exam Description. This is going to be mostly informative, but this is good information to know before going into your exam. It’s always a good idea to understand the expectations of those giving standardized tests like the APs.
– Take a look at the videos “pH and Buffers,” Solubility,” and “Free Energy and the Equilibrium Constant” from Bozeman Science’s AP® Chemistry page, but do so alongside reading to the end of your textbook. Which style of learning did you find more favorable? If you liked the videos better, you may be a visual learner and should review the charts and graphs provided by these materials, they may be of more benefit to you.
– Review all of your flashcards and notes. Polish up those final weak spots you see and be proud that you’re getting better at AP® Chemistry.
– Read the Albert.io article LeChatelier’s Principle Review.
– Go ahead and finish the remaining example questions from Gibbs Free Energy and Equilibrium. After you have done this, read over your notes on the example questions and the FRQs. Retake those questions that you feel the least comfortable with or that you seemed to not quite understand.
Day 30
– You did it! You made it to the last day: congratulations.
– Today you will finish with a practice exam from The CollegeBoard website. Make sure you read through the instructions. Be strict about time limits here and definitely no notes. Take this practice exam as if it was the real thing.
– After you have finished taking and grading the practice exam, put everything away. Again, try to clear your mind. You should feel confident and refreshed for your upcoming test.
– The confidence should come from the hours you’ve spent studying at home, school, and in the lab. If you have kept up with this daily study guide, you will have:
– Notes, key terms, and flashcards on every section of the course.
– A deep and thorough understanding of the six Big Ideas the make up the AP® Chemistry course guidelines.
– Observed dozens of visual and hands-on examples of chemical processes through expansive informational videos and laboratory experiments.
– Completed a broad range of example Free Response Questions and practiced with over a thousand multiple choice questions!
For however much time you still have after completing this AP® study, make sure to periodically review your notes, key terms, flashcards, and timeline.
You’ll also want to make sure to keep yourself healthy and confident. If you enjoyed the study break, keeps going on your walks, openly thinking about what you have learned from this study plan. On the night before the exam itself, eat a healthy dinner and get plenty of sleep. On the day of the exam make sure you have a good breakfast to keep you energized.
Finally, maintain that feeling of confidence. The fact that you completed this comprehensive 30-day study guide shows your dedication and drive. Keep up with the hard work and good luck!
Let us know what has worked for you. What did you like best about this one month study guide? Do you have recommendations of your own on how to study for the AP Chemistry exam?
Start your AP® Chemistry Prep today
Looking for AP® Chemistry practice?
Kickstart your AP® Chemistry prep with Albert. Start your AP® exam prep today.









2 thoughts on “One Month AP® Chemistry Study Guide”
Thank you!
You’re welcome!
Comments are closed.