What We Review
Introduction
Osmosis and tonicity are central concepts in biology that describe how water moves across cell membranes in response to different solute concentrations. A solid understanding of these concepts is crucial not only for success in the AP® Biology exam but also for grasping how cells maintain homeostasis. In this post, we will explore the basics of osmosis, distinguish among different types of tonicity, and discuss key factors like osmolarity that determine the movement of water in living organisms.
Understanding Osmosis
A. Definition of Osmosis
Osmosis is the passive movement of water molecules across a semipermeable membrane from an area of higher water potential (or lower solute concentration) to an area of lower water potential (or higher solute concentration). Because it requires no energy input from the cell, osmosis is a type of passive transport.
B. The Role of Concentration Gradients
Water movement during osmosis is driven by concentration gradients. When solute concentrations differ on either side of a membrane, a gradient is created. Water flows toward the side with higher solute concentration to dilute it until an equilibrium is reached. In biological systems, substances like salts, sugars, and ions play a major role in establishing these gradients.
Tonicity and its Types
A. What is Tonicity?
Tonicity describes how a surrounding solution affects a cell’s volume by the net flow of water. This concept helps us predict whether a cell will swell, shrink, or remain stable when placed in different environments.
B. Types of Tonic Environments
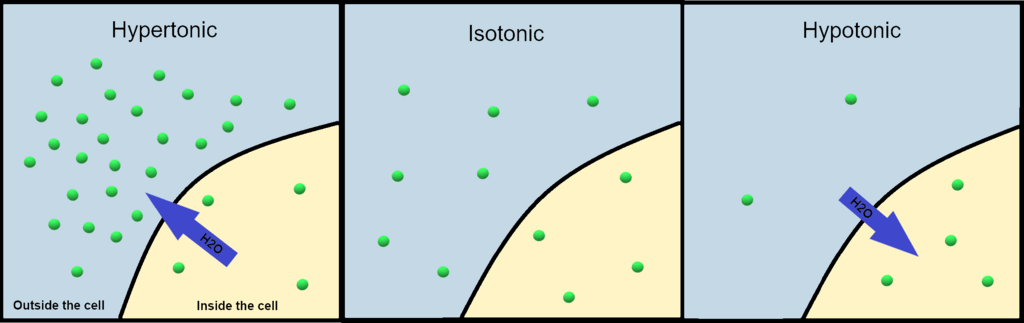
- Hypotonic
- Definition and Characteristics: A hypotonic solution has a lower solute concentration compared to the inside of the cell.
- Effects on Cells: Water moves into the cell, causing it to swell. In animal cells, excessive swelling can lead to cell lysis (bursting). Plant cells, however, are protected by a rigid cell wall and generally prefer slightly hypotonic environments that keep them turgid.
- Hypertonic
- Definition and Characteristics: A hypertonic solution has a higher solute concentration outside than inside the cell.
- Effects on Cells: Water flows out of the cell, causing it to shrink or crenate. In plant cells, this leads to plasmolysis, where the cell membrane pulls away from the cell wall.
- Isotonic
- Definition and Characteristics: An isotonic solution has an equal solute concentration on both sides of the membrane.
- Effects on Cells: There is no net movement of water. Cells maintain their normal shape. This is the ideal condition for animal cells, which require balanced solute levels to function properly.
Osmolarity: A Key Component
Osmolarity refers to the total solute concentration in a solution. It is closely related to tonicity because it influences how water moves across cell membranes. Higher osmolarity means more solute particles in a solution, which generally results in lower water potential and a greater pull for water movement into that area.
Mechanisms of Osmoregulation
A. Importance of Osmoregulation
Organisms must regulate their internal solute and water balance to survive in varying environmental conditions. Cells that fail to maintain proper water balance can become damaged or die.
B. Homeostasis and Water Balance
- Animals: Many animals rely on specialized organs (like kidneys) or behaviors (such as seeking fresh water) to regulate osmolarity.
- Plants: Plants control water balance through structures such as stomata, allowing them to regulate water loss and uptake.
- Single-celled organisms: Protists, for example, use contractile vacuoles to expel excess water and maintain osmotic balance.
Examples and Applications
- Red Blood Cells (RBCs): When placed in a hypotonic solution (e.g., pure water), RBCs swell and may burst, while in a hypertonic solution (e.g., highly salted water), they shrink. An isotonic solution (similar to physiological saline) keeps RBCs at their normal shape.
- Medical Saline Solutions: In hospitals, saline solutions are typically isotonic to prevent damage to blood cells.
- Plant Cells in Freshwater: Many freshwater plants thrive in a slightly hypotonic environment, which provides turgor pressure and structural support.
Practice Problems
- A red blood cell is placed in a solution that causes it to shrink. Which term best describes the solution, and why?
- A plant cell is very firm and does not wilt. Is it likely in a hypotonic, hypertonic, or isotonic environment? Explain.
- Two solutions with different solute concentrations are separated by a semipermeable membrane. Explain how water moves until equilibrium is reached.
Sample Answers:
- The solution is hypertonic. Since it has a higher solute concentration, water leaves the cell to dilute the external environment, causing the cell to shrink.
- A firm, turgid plant cell is in a hypotonic environment, which allows water to enter the cell and create internal pressure against the cell wall.
- Water moves from the region of lower solute concentration (higher water potential) to the region of higher solute concentration (lower water potential) until both sides have an equal concentration of solutes.
Conclusion
Osmosis and tonicity are crucial for understanding cell behavior, physiology, and survival strategies in various environments. Mastering these concepts will not only help you excel in AP® Biology but will also deepen your appreciation of the remarkable ways organisms regulate and maintain their internal environments.
Sharpen Your Skills for AP® Biology
Are you preparing for the AP® Biology test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world math problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
Need help preparing for your AP® Biology exam?
Albert has hundreds of AP® Biology practice questions, free response, and full-length practice tests to try out.