Properties of Water Introduction
On the AP® Biology exam, many students struggle with questions that relate to the properties of water. This topic is usually covered at the beginning of the year, and when it comes to all the information students are responsible in AP® Bio, it gets forgotten. The properties of water are essential to maintaining homeostasis, a stable equilibrium, in organisms and in the world.
In this AP® Biology Crash Course Review, we will review the information that is essential for you to understand about water properties. We will begin by talking about the polarity of water and how its polarity lends itself to making water one of the most important chemicals on Earth. We will then talk about several important properties that water has that allow organisms to survive. Finally, we will review a free response question about the properties of water that students were given on the 2009 AP® Biology exam.
The Importance of Polarity & Hydrogen Bonds
At this point in your science career, you have probably learned about polarity. A polar molecule is a molecule that does not share its electrons equally between its atoms. This electron imbalance causes the molecule to have positive and negative dipoles; because the electrons are spending more time at one end (due to unequal sharing), this part of the molecule will have a partial negative charge. The end that is pulling the electron towards it will be the negative dipole, and the end getting the electron pulled away from it will be the positive dipole.
The amount of force that the negative atom exerts is called electronegativity. It is important to understand that oxygen has one of the highest electronegativity values, which means that it will pull the electrons away from the atom that it is connected to, making it the negative dipole. In the case of water, oxygen will pull the electron from hydrogen. Keep in mind that water molecules are still sharing their electrons, but the electrons spend a longer amount of time with the oxygen than with the hydrogen.
Why is all of this important? The dipoles end up making the oxygen atom negatively charged and the hydrogen atoms positively charged. Water is made up of millions of these molecules, which cause them to orient themselves in a certain way. The negative part of one water molecule will want to be near the positive part of another. This grid-like bonding is called hydrogen bonding.
Hydrogen bonding is one of the most important things that you must know about water. Hydrogen bonding is the reason for the properties of water that you must know for your AP® Bio exam. For every property that we discuss, we will review how it occurs due to hydrogen bonding and how that affects life on Earth.
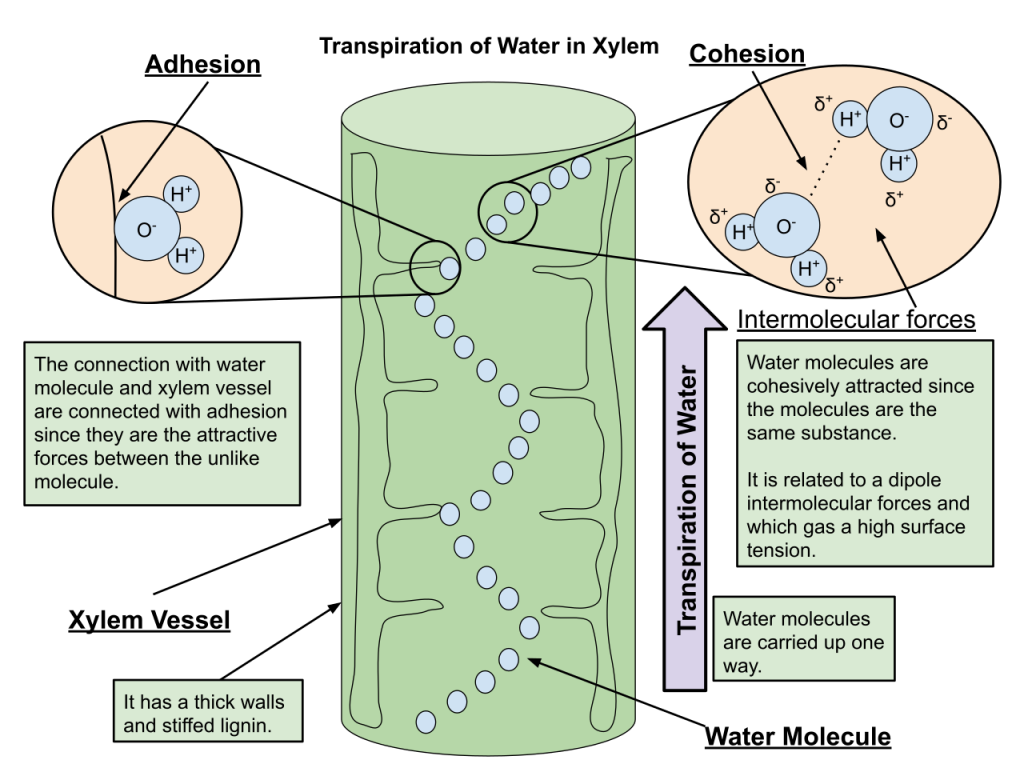
Adhesion
The first two properties that we will discuss in our AP® Biology Crash Course Review are related to each other: adhesion and cohesion. Adhesion is the property of water that refers to water molecules sticking to another surface. Cohesion is the property of water that refers to water molecules sticking to each other. Both of these properties are due to hydrogen bonding and how hydrogen bonding orients the water molecules.
Adhesion is an important property. To demonstrate adhesion, fill a test tube with water. You will see the water create a U. This U is called the meniscus. The meniscus is formed because the water is sticking to the glass of the test tube. If you have an even skinnier test tube, you will see that the meniscus will get deeper (the water will crawl higher). If you don’t have a test tube, please note the illustration.
Adhesion occurs naturally as well and is the reason that trees can be tall. Trees get their water from their roots. Roots are far away from the leaves that need the water. The xylem is the part of the tree that water moves up, against gravity, to get to the leaves. The xylem is made up of little capillaries. When water enters into the xylem, the water will adhere to the sides of the capillary and allow it to move to the leaves. This process is called capillary action and is vital to the survival of plants.
Cohesion
Cohesion, as we have introduced, is water’s ability to bind to itself. Cohesion is responsible for surface tension, which means that water droplets will resist rupture when stress and pressure are added to the system.
Cohesion occurs when water is surrounded by air. The molecules that exist on the ends of droplets have fewer opportunities to hydrogen bond. This causes their hydrogen bonds to strengthen; the surface is able to withstand tension.
Cohesion is an essential property of water for many insect species. An example is found in the Gerridae family of insects. These insects are commonly called water striders. They are able to use the surface tension of water to stand on the water. The habitat of water striders is the surface of calm waters. It is here where they hunt and reproduce. Without the cohesive properties of waters, these insects would not survive as they do now.

Specific Heat Capacity
Due to the strength of hydrogen bonds, water has an extremely high specific heat capacity. Specific heat refers to the amount of heat necessary to move a substance up one degree Celsius. A high specific heat capacity means that water is able to withstand a high amount of energy before its temperature is changed; this is due to water’s hydrogen bonding. In order for water to be heated up, hydrogen bonds must be broken. This additional energy needed to break the bonds gives water the ability to withstand temperature changes.
There are two important temperatures that are often studied in compounds: heat of vaporization and heat of fusion. Heat of vaporization refers to the heat needed to move a compound from liquid state to gas state. Heat of fusion refers to the heat needed to move a compound form the solid state to liquid state. Due to the hydrogen bonds, water has both high heat of vaporization and high heat of fusion.
The high specific heat is extremely important to the survival of organisms on Earth. Earth is made up of 70% water. The water on the planet prevents the temperature from radically changing. When the temperature changes in the atmosphere, water absorbs the change in temperature, preventing organisms from having to survive in dynamic environments. Additionally, humans (like most organisms), have bodies with very high water contents. For the very same reason, this water content prevents body temperature from fluctuating.
Ice Density
This may seem like the most obvious property of water; water in its solid form floats in its liquid form. The majority of substances found on Earth have a much denser solid version than liquid version, which should make intuitive sense. When a substance becomes solid, it condenses, making the bonds closer and stronger. Why then does water disobey this rule and float? You probably guessed it, hydrogen bonds.
The hydrogen bonds in water cause solid water to form a three-dimensional matrix. The hydrogen bonds keep the bonds spread out, not allowing water to condense, but rather forcing it to spread. When water is in a liquid state, hydrogen bonds are much more flexible and able to move around. When water becomes a solid, the bonds become less flexible, causing the water to expand. The expansion of the bonds causes the ice’s density to decrease; less dense compounds will float in more dense compounds.
Other than being interesting, this is extremely vital to our survival on Earth. If water sank when it was in its solid form, because the ice density was lower, our planet would be frozen. If water sank when it was exposed to frigid temperatures, the next layer of water would sink as well until the entire ocean was frozen solid. Not only would marine animals die, but terrestrial organisms would likely not survive either.
Universal Solvent

You may have heard that water is the universal solvent. Water is considered the universal solvent, because it is able to dissolve compounds very quickly. This property is unique to water because of the dipoles that water molecules have.
When a compound is introduced to water, molecules of water will surround it, forming a cage around the molecules of the compound. For example, when NaCl (table salt) is placed in water, Na is ripped from Cl. Water molecules rip apart the compound, because positively charged hydrogen molecules want to bond with the negatively charged chlorine ion. Additionally, the negatively charged oxygen dipole wants to bond with the positively charged sodium ion. This attraction forces the molecules apart and causes water to dissolve salt rapidly.
There is a class of compounds, which water will not be able to dissolve. These compounds are nonpolar compounds. Nonpolar compounds share their electrons equally and do not have charges or dipoles. Because of this characteristic, water will not be able to pull apart the different molecules. This is the reason why oil (nonpolar) and water (polar)will not mix.
Solvents are incredibly necessary for many reasons. We are constantly ingesting food that must be broken down quickly and efficiently. The presence of water in our bodies aids in our digestion. Solvents are important in nearly every chemical reaction.
Summary
In this AP® Biology Crash Course Review we have talked about the many different properties of water that allow life on Earth. We talked about cohesion, which allows for insects like water striders to stand on water. We reviewed adhesion as well, which allows water to travel up to the leaves of a tree. We then learned about water’s high specific heat and how that allows the temperature on Earth and in organisms, like humans, to stay at homeostasis. Next, we talked about how water’s solid form, ice, floats and how that has allowed our oceans to remain thawed. Finally, we learned about how water is a universal solvent capable of dissolving any polar substance.
There has been a lot of information to review in this article, Water Properties: AP® Biology Crash Course Review. To make sure that you feel comfortable, we will now review an AP® Bio question about water properties that was seen on the AP® exam in 2009.
AP® Biology Exam Question
Water is essential to all living things.
(a) Discuss THREE properties of water.
(b) Explain each of the following in terms of the properties of water. You are not limited to the three properties discussed in part (a):
- the role of water as a medium for the metabolic processes of cells
- the ability of water to moderate temperature within living organisms and in organisms’ environments
- the movement of water from the roots to the leaves of plants
For part A, you have choices. Here are a few of the answers that the AP® Biology Exam would accept for full points:
| Property | Description |
| Cohesion | Attraction to other water molecules, surface tension |
| Adhesion | Attraction to other surfaces |
| High Specific Heat | Heat absorption without temperature change |
| States of Matter | Ice is less dense than water, expands and becomes less dense |
| High heat of vaporization | Water molecules absorb energy |
For part B, you must explain the three important parts of life on Earth and how water is necessary for their existence. The first example refers to water’s ability to be a universal solvent. We reviewed that it is important that water is able to dissolve substances, and in a cell, this is especially important. Water’s presence also allows for many chemical reactions to occur.
The second example we reviewed previously in this article. Water moderates temperature by absorbing heat and not changing temperature. This allows for the climate to remain more stable. Water’s high specific heat will also allow for constant internal environments within organisms.
Finally, the third example we can use the capillary action of water. Water moves up the xylem of the tree in order to reach the leaves. Water moves using adhesion and capillary action.
Thank you so much for reading our article! Let us know if you have any questions or if you want to share how you are studying for your AP® Bio exam below. If you want to keep studying, check out our article Homeostasis: AP® Biology Crash Course Review now!
Need help preparing for your AP® Biology exam?
Albert has hundreds of AP® Biology practice questions, free response, and full-length practice tests to try out.
Are you a teacher or administrator interested in boosting AP® Biology student outcomes?
Learn more about our school licenses here.








