Pressure is a fundamental concept in physics, illustrating how force is distributed over an area. For AP® Physics 1 students, grasping the concept of pressure is crucial, as it serves as a building block for more advanced topics. This article will explore the definition of pressure, the pressure formula, its units, and practical applications while providing easy-to-follow examples and explanations.
What We Review
What is the Pressure Formula?
Pressure measures how much force is applied over a specific area. Imagine pressing a nail into a wooden board with the same force as your palm. The nail penetrates easily because the force is concentrated over a small area, demonstrating high pressure. On the other hand, your palm applies force over a larger area, resulting in lower pressure.
The formula for calculating pressure is:
P = \dfrac{F_{\perp}}{A}…where:
- P is pressure
- F_{\perp} is the perpendicular force
- A is the area.
It’s important to note that pressure is a scalar quantity, meaning it has magnitude but no direction.
Understanding Gauge Pressure and Absolute Pressure
On the AP® Physics 1 exam, you will be asked to distinguish between gauge pressure and absolute pressure. Gauge pressure measures the pressure relative to atmospheric pressure, commonly used in tire pressure and other applications. The gauge pressure formula is:
P_{\text{gauge}} = \rho gh…where:
- \rho is the fluid density,
- g is the acceleration due to gravity,
- h is the height of the fluid column.
Absolute pressure considers both the gauge pressure and atmospheric pressure, calculated by:
P = P_0 + P_{\text{gauge}}Where P_0 is atmospheric pressure.
Units of Pressure
Pressure is measured in several units, so ensuring the correct unit is vital in calculations. Common pressure units include:
- Pascal Pa — the standard SI unit
- Bar — 1 bar = 100{,}000 \text { Pa }
- Atmosphere (\text { atm }) — 1 \text { atm } = 101{,}325 \text{ Pa }
Let’s see an example of unit conversion:
Example: Converting Pressure Units
Convert 500,000 Pa to atm.
- Understand the conversion factor: 1 \, \text{atm} = 101{,}325 \, \text{Pa}
- Use the formula: \text{Pressure in atm} = \frac{500{,}000 \, \text{Pa}}{101{,}325 \, \text{Pa/atm}}
- Calculate: \approx 4.93 \, \text{atm}
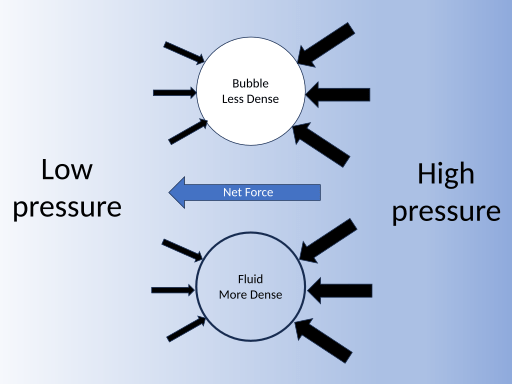
How Pressure Works in Fluids
Fluids, such as liquids and gases, behave uniquely under pressure. In incompressible fluids, like water, density remains constant, and pressure depends on depth. As such, deeper water exhibits more pressure.
Example: Exploring Pressure in Fluids
Calculate the pressure at the base of a 10 m tall water column.
- Use the formula for gauge pressure: P_{\text{gauge}} = \rho gh
- Insert known values:
- Water density \rho = 1000\text{ kg/m}^3
- Gravitational acceleration g = 9.81\text{ m/s}^2
- Height h = 10\text{ m}.
- Calculate: P_{\text{gauge}} = 1000 \times 9.81 \times 10 = 98{,}100 \, \text{Pa}
Real-World Applications of Pressure
Understanding pressure is vital in various practical applications:
- Hydraulics: Devices like car brakes use fluid pressure to transmit force.
- Barometers: Measure atmospheric pressure, essential for weather predictions.
For instance, hydraulic systems rely on Pascal’s Principle, where pressure applied to a confined fluid is transmitted undiminished throughout the fluid. This principle allows a small force exerted on a small area to generate a large force over a larger area.
Worked Examples
Example 1: Calculating Gauge Pressure
A diver swims 5 meters under the sea. Calculate the gauge pressure exerted on the diver by seawater (seawater density = 1030 kg/m³).
- Use the gauge pressure formula: P_{\text{gauge}} = \rho gh
- Insert known values:
- Density \rho = 1030\text{ kg/m}^3
- Gravitational acceleration g = 9.81\text{ m/s}^2
- Depth h = 5\text{ m}
- Calculate: P_{\text{gauge}} = 1030 \times 9.81 \times 5 = 50{,}535 \, \text{Pa}
Example 2: Calculating Absolute Pressure
Continue from Example 1. The atmospheric pressure at sea level is 101,325 Pa. Calculate the absolute pressure on the diver.
- Use the absolute pressure formula: P = P_0 + P_{\text{gauge}}
- Insert known values:
- Atmospheric pressure P_0 = 101{,}325\text{ Pa}
- Gauge pressure P_{\text{gauge}} = 50{,}535 \text{ Pa}.
- Calculate: P = 101{,}325 + 50{,}535 = 151{,}860 \, \text{Pa}
Conclusion
A strong grasp of pressure is crucial for success in AP® Physics 1, as it applies to fluids, gases, and real-world engineering systems. Whether analyzing gauge pressure, absolute pressure, or Pascal’s principle, understanding these concepts builds a solid foundation for more advanced physics topics.
Key Takeaways:
- Pressure Formula:P = \frac{F}{A} – Pressure depends on the applied force and surface area.
- Gauge vs. Absolute Pressure: Learn when to add atmospheric pressure to calculations.
- Practice is Key: Solve problems, apply concepts to real-world situations, and experiment with fluid pressure scenarios.
To excel in AP® Physics 1, reinforce your understanding through problem-solving, simulations, and hands-on experiments. The more you explore, the more confident you’ll become in applying pressure concepts to physics and beyond!
| Term | Definition |
| Pressure (P) | Force per unit area. Measure of force distribution. |
| Pascal (Pa) | SI unit of pressure, equal to one newton per square meter. |
| Gauge Pressure | Pressure relative to atmospheric pressure. |
| Absolute Pressure | Total pressure, including atmospheric pressure. |
| Atmosphere (atm) | Unit, 1 \text{atm} = 101,325 Pascal. |
Sharpen Your Skills for AP® Physics 1
Are you preparing for the AP® Physics 1 test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world physics problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
Need help preparing for your AP® Physics 1 exam?
Albert has hundreds of AP® Physics 1 practice questions, free response, and full-length practice tests to try out.








