Deoxyribonucleic acid, fondly known as DNA, is a molecule in the shape of a double helix. DNA is responsible for storing genetic information in the cells of all living organisms. But what is DNA made of exactly? In this post, we will review DNA and nucleotides.
What is a Nucleotide?
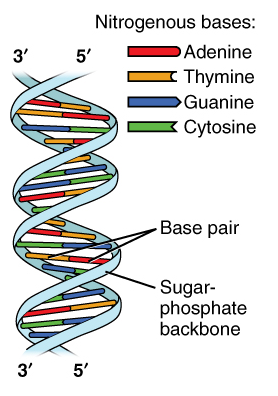
Image Source: Wikimedia Commons
Figure 1: The double-helix of the DNA
DNA, and other nucleic acids such as RNA, are made up of nucleotides. Nucleotides are the building blocks of DNA and RNA. The structure of DNA can be visualized or thought of as a ladder. Each “step or rung” of this ladder is made up of a string of nucleotides, in a very specific and controlled order. Each nucleotide, in turn, is made up of a nitrogenous base, a pentose sugar, and a phosphate. This particular molecule is adenine; we will find out more about this later.
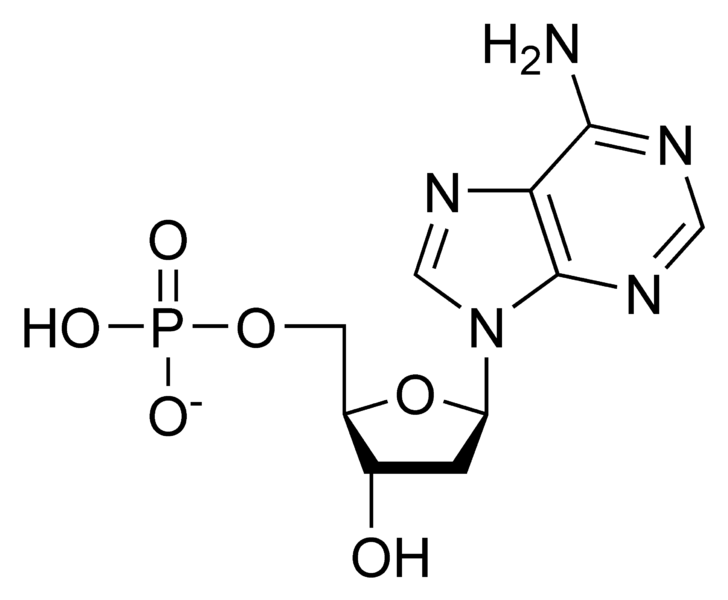
Image Source: Wikimedia Commons
Figure 2: The chemical assembly of the three parts of the nucleotide, the phosphate, the nitrogenous base, and the pentose sugar. This particular nucleotide is adenine
The assembly of nucleotides has three key purposes. First, it differentiates them from nucleosides, which do not contain a phosphate group. Next, it allows the nucleotide to connect to other nucleotides. This occurs when the nitrogenous base forms a hydrogen bond with another nucleotide’s nitrogenous base. Lastly, it allows the phosphate to form a phosphodiester bond with another nucleotide’s pentose sugar.
As a result, this assembly creates a complex double-stranded “string or ladder”, as seen in Figure 1. This is the basis of the form of DNA.
What We Review
The Nitrogenous Base
The word “nucleotide” was first coined by P.A. Levene. Levene observed that DNA contained four similar building blocks, in roughly equal amounts. Specifically, these building blocks are what we now know as the nitrogenous bases found in DNA and RNA.
A nitrogenous base is a molecule containing nitrogen, with the chemical properties of a base due to a pair of electrons on the nitrogen atom. These nitrogenous bases are Adenine (A), Cytosine (C), and Guanine (G) which are found in both RNA and DNA, and then Thymine (T) which is only found in DNA. In RNA, Uracil (U) takes the place of Thymine.
Pyrimidines and Purines
Nitrogenous bases can be further classified as pyrimidines or purines. Cytosine, uracil, and thymine are all pyrimidines. That is, their molecular structure comprises a nitrogenous base in the form of a six-member single ring. Guanine and adenine, on the other hand, are purines. These contain a nitrogenous base in the form of a nine-member double ring. In short, pyrimidines have only one ring while purines have two (figure 3).
Now that you get the general idea of purines versus pyrimidines let’s speak biochemistry. A purine is a heterocyclic aromatic organic compound that comprises a pyrimidine ring that is joined to an imidazole ring. The next logical question, of course, becomes “What then is a pyrimidine, biochemically speaking”? Well, pyrimidines are a class of nitrogenous compounds that have only one heterocyclic ring.
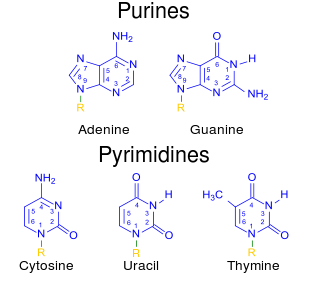
Image Source: Wikimedia Commons
Figure 3: Chemical structure of purines (A, G) and pyrimidines (C, T/U)
Base Pairs
Nitrogenous bases form base pairs with each other in DNA. Adenine always pairs with thymine; guanine is always bonded to cytosine. You’ll also notice that this means that a pyrimidine is always bonded to a purine. The bond formed is a hydrogen bond, and is responsible for the rungs formed in the DNA “ladder”. This architecture is very important for the perfect construction of the DNA molecule. Otherwise, there would be bumps and crevices on the molecule. This wouldn’t do at all because the very careful packaging, unwinding, and winding of the DNA would be a mess with some more difficult to maintain than others.
This pairing is, therefore, crucial for genetic function, and is the foundation for DNA replication and gene expression. The order in which base pairs appear determines the functioning of your physiology. In protein synthesis, for example, the code is read in triplicates where three bases code for a particular amino acid. Deletions and insertions of nucleotides in this situation can lead to a complete frame shift disrupting the synthesis of the protein in question. Substitutions can also be problematic although less so, as they may change the identity of an amino acid in the protein code.
The Phosphate Group
The phosphate group (PO4) is what differentiates a nucleotide from a nucleoside. This addition changes the nucleoside from a base to an acid. These phosphate groups are important, as they form phosphodiester bonds with the pentose sugars to create the sides of the DNA “ladder”. This is critical, as the hydrogen bonds which join the nitrogenous bases are not very strong. These sides of the ladder are hydrophilic (attracted to water) and therefore allow the DNA molecule to bond with water.
What are Nucleoside Diphosphates and Triphosphates?
You know that a nucleotide is differentiated from a nucleoside by one phosphate group. Accordingly, a nucleotide can also be a nucleoside monophosphate(figure 4). If more phosphates bond to the nucleotide (nucleoside monophosphate), it can become a nucleoside diphosphate (if two phosphates bond), or a nucleoside triphosphate (if three phosphates bond), such as adenosine triphosphate (ATP). ATP is a crucial component of respiration and photosynthesis, amongst other processes.
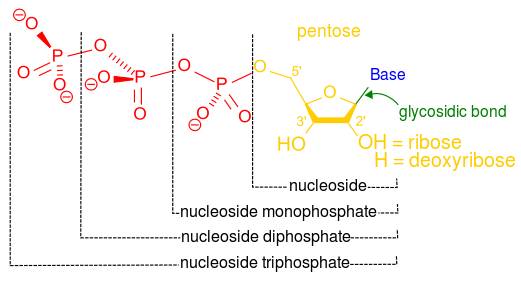
Image Source: Wikimedia Commons
Figure 4: The molecular structure of nucleoside mono-, di- and triphosphate
A polynucleotide is a chain of more than 20 nucleotides joined by a phosphodiester bond.
The Pentose Sugar
The pentose sugar is a 5-carbon monosaccharide with the formula (CH2O)5. These form two groups: aldopentoses and ketopentoses. The pentose sugars found in nucleotides are aldopentoses. Deoxyribose and ribose are two of these sugars.
These sugars differ in DNA and RNA. The sugar in DNA is deoxyribonucleic acid, which contains deoxyribose. The sugar in RNA is ribonucleic acid, which contains ribose. The structural difference between these sugars is that ribonucleic acid contains a hydroxyl (-OH) group, whereas deoxyribonucleic acid contains only a hydrogen atom in place of this hydroxyl group. Deoxyribonucleotides are nucleotides that contain deoxyribonucleic acid. Meanwhile, those containing ribonucleic acid are known as ribonucleotides. Thus, the sugar molecule determines whether a nucleotide forms part of a DNA molecule or a RNA molecule. The table below lists the names given to the sugars found in RNA and DNA.
Base |
Ribonucleoside |
Ribonucleotide |
Deoxyribonucleoside |
Deoxyribonucleotide |
A | Adenosine | Adenylic acid | Deoxyadenosine | Deoxyadenylic acid |
C | Cytidine | Cytidylic acid | Deoxycytidine | Deoxycytidylic acid |
G | Guanosine | Guanylic acid | Deoxyguanosine | Deoxyguanylic acid |
U | Uridine | Uridylic acid | ||
T | Deoxythymidine | Deoxythymidylic acid |
Putting it All Together
To summarize, we have covered what a nucleotide is and what the three parts of a nucleotide are. We have also covered the specifics of nitrogenous bases, pentose sugars, and phosphates, and we have discussed how nucleotides differ in DNA and RNA.
The phosphate connects to the pentose sugar; the pentose sugar connects to the nitrogenous base pair (A, C, G or T), which in DNA connects to its base pair partner. Something like this:
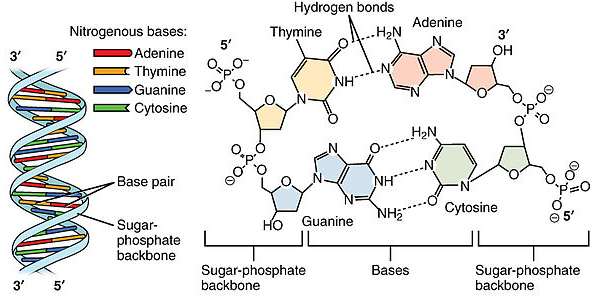
Image Source: Wikimedia Commons
Figure 5: Nucleotide bonding in the DNA molecule with hydrogen and phosphate bonds.
The chemical structure of the phosphate, pentose sugar, and nitrogenous bases of adenine, thymine, cytosine, and guanine are shown above (Figure 5).
During DNA synthesis, a hydrogen bond joins A (adenine) to T (thymine), and C (cytosine) to G (guanine) (figure 5). In RNA, uracil would replace thymine.
A DNA strand is formed when the nitrogenous bases are joined by hydrogen bonds, and the phosphates of one group are joined to the pentose sugars of the next group with a phosphodiester bond (Figure 5).
The double helix shape is the result of the hydrogen bonds between the nitrogen bases. These form the “rungs” of the ladder while the phosphate and pentose sugar (forming phosphodiester bonds) form the upright parts of the ladder.
Conclusion
To conclude, nucleotides are important as they form the building blocks of nucleic acids, including DNA and RNA. Nucleotides consist of 3 parts. The first is a distinct nitrogenous base, which is adenine, cytosine, guanine or thymine. In RNA, uracil replaces thymine. These nitrogenous bases are either purines or pyrimidines. Base pairs form when adenine forms a hydrogen bond with thymine, or cytosine forms a hydrogen bond with guanine.
The second part of a nucleotide is the phosphate, which differentiates the nucleotide molecule from a nucleoside molecule. This phosphate is important in the formation of phosphodiester bonds, which link several nucleotides in a linear fashion.
Lastly, the third part of a nucleotide is the pentose (5 carbon) sugar. The pentose sugars found in nucleotides are aldopentoses: ribose in RNA and deoxyribose in DNA. These sugars determine whether the nucleotide will form part of a DNA or a RNA molecule, and form part of the phosphodiester bonds which link several nucleotides. The combination of hydrogen bonds between nitrogenous bases and phosphodiester bonds between phosphates and sugars is what gives DNA its double helix shape.
Looking for more Cellular and Molecular Biology practice?
Check out our other articles on Cellular and Molecular Biology.
You can also find thousands of practice questions on Albert.io. Albert.io lets you customize your learning experience to target practice where you need the most help. We’ll give you challenging practice questions to help you achieve mastery of Biology.








