What We Review
Introduction
Monomers may be tiny, but they play a powerful role in making up the biological macromolecules that keep life going. Even if the term “monomer” sounds abstract, it’s one of the fundamental building blocks you’ll need to understand for the AP® Biology exam. In this post, we’ll explore what monomers are, how they connect to form larger molecules, and why these concepts matter in biology.
What is a Monomer?
Definition of a Monomer
A monomer is a small molecule that can bond with identical or similar molecules to form polymers. Think of them as the individual Lego bricks that, when linked together, build the final structure—be it a protein, a nucleic acid, or a carbohydrate.
Examples of Common Monomers
- Amino Acids → the monomers that link together to form proteins.
- Nucleotides → the building blocks of nucleic acids (DNA and RNA).
- Monosaccharides (e.g., glucose) → the monomers that combine to form complex carbohydrates (like starch or glycogen).
The Role of Monomers in Biological Macromolecules
Biological macromolecules—proteins, nucleic acids, carbohydrates, and lipids—owe their structure and function to combinations of monomers:
- Proteins: Long chains of amino acids fold into specific shapes that allow them to act as enzymes, transport molecules, and more.
- Nucleic Acids: DNA and RNA carry genetic information, thanks to various arrangements of nucleotides.
- Carbohydrates: Sugars link together into short or long chains (like starch or cellulose) to provide energy and structural support.
- Lipids: While not always assembled in the same linear chains as proteins or carbs, certain lipids do form from smaller repeated units, giving them unique structural properties.
By understanding monomers and how they come together, you’ll gain insight into how these larger molecules function in cells.
Types of Bonds Connecting Monomers
Definition of Covalent Bonding
Covalent bonds are strong chemical bonds formed when two atoms share one or more pairs of electrons. These are the primary bonds that connect monomers to form polymers. For example, a covalent peptide bond links one amino acid to another in a protein chain.
- Peptide Bonds: Connect amino acids in proteins.
- Phosphodiester Bonds: Link nucleotides in DNA or RNA.
- Glycosidic Bonds: Join monosaccharides in carbohydrates.
Understanding how covalent bonds work will help you see why some biological molecules are stable and can form complex structures.
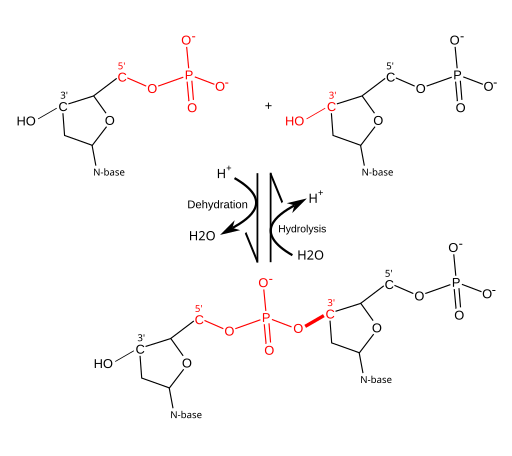
Synthetic Processes: Dehydration Synthesis and Hydrolysis
Dehydration Synthesis
- Definition and Process: In dehydration synthesis (also known as condensation reaction), a water molecule (H₂O) is removed (dehydration) when two monomers join (synthesis).
- Formation of Covalent Bonds: The removal of -OH (hydroxyl group) from one monomer and -H (hydrogen) from another creates space for a new covalent bond.
- Example: When two glucose molecules link, they release one water molecule and form a disaccharide like maltose.
Hydrolysis
- Definition and Process: Hydrolysis is essentially the reverse of dehydration synthesis. It uses a water molecule to break a covalent bond, splitting a polymer back into its monomers.
- Breaking Covalent Bonds: An -OH and an -H from water attach to the resulting fragments, separating them.
- Example: Digestion of proteins in your body uses hydrolysis reactions to break large protein molecules into individual amino acids.
Comparative Overview: Dehydration Synthesis vs. Hydrolysis
- Dehydration Synthesis
- Builds larger molecules (polymers from monomers).
- Water is released as a byproduct.
- Formation of covalent bonds.
- Hydrolysis
- Breaks larger molecules into smaller units (polymers into monomers).
- Requires water to cleave the bond.
- Essential in digestion and recycling of biomolecules.
Both processes are crucial for the constant building up and breaking down of molecules within organisms. Life relies on this balance to maintain homeostasis.
Practical Examples and Applications
- Genetics: DNA replication and RNA transcription rely on nucleotide monomers linking together. Understanding monomers helps clarify how cells copy and utilize genetic information.
- Biochemistry: Many enzymes (proteins) catalyze dehydration synthesis or hydrolysis reactions in processes like digestion or metabolism.
- Molecular Biology: Research on how certain polymers form can lead to medical advancements, like developing new drugs that inhibit or enhance these reactions.
By seeing these concepts in action, you can recognize why mastering monomers and their bonding mechanisms is essential—not only for the AP® Biology exam but also for future scientific exploration.
Conclusion
Monomers are the starting point for understanding how complex biological molecules come together. Whether you’re looking at the structure of proteins or exploring the double helix of DNA, recognizing the role of these small building blocks makes bigger concepts clearer. Study the details of covalent bonds, dehydration synthesis, and hydrolysis, and you’ll be prepared to tackle test questions and real-world applications alike.
Remember: Proteins, nucleic acids, and carbohydrates wouldn’t exist without their monomer “Lego pieces.” By mastering these fundamentals, you set yourself up for success on the AP® Biology exam—and beyond.
Practice Questions
- Define the term “monomer” and give one biological example besides amino acids or nucleotides.
- Explain how a covalent bond differs from an ionic bond in the context of forming polymers.
- Describe how dehydration synthesis and hydrolysis reactions could be viewed as opposite processes.
- A student explains that “lipids are not formed by monomers.” In what way is this statement incomplete or misleading?
- Provide a real-world biological example where hydrolysis is critical for the organism’s survival.
By answering these questions, you’ll strengthen your understanding of monomers, bonding, and the essential processes like dehydration synthesis and hydrolysis that you’ll encounter in AP® Biology. Good luck studying!
Sharpen Your Skills for AP® Biology
Are you preparing for the AP® Biology test? We’ve got you covered! Try our review articles designed to help you confidently tackle real-world math problems. You’ll find everything you need to succeed, from quick tips to detailed strategies. Start exploring now!
Need help preparing for your AP® Biology exam?
Albert has hundreds of AP® Biology practice questions, free response, and full-length practice tests to try out.